
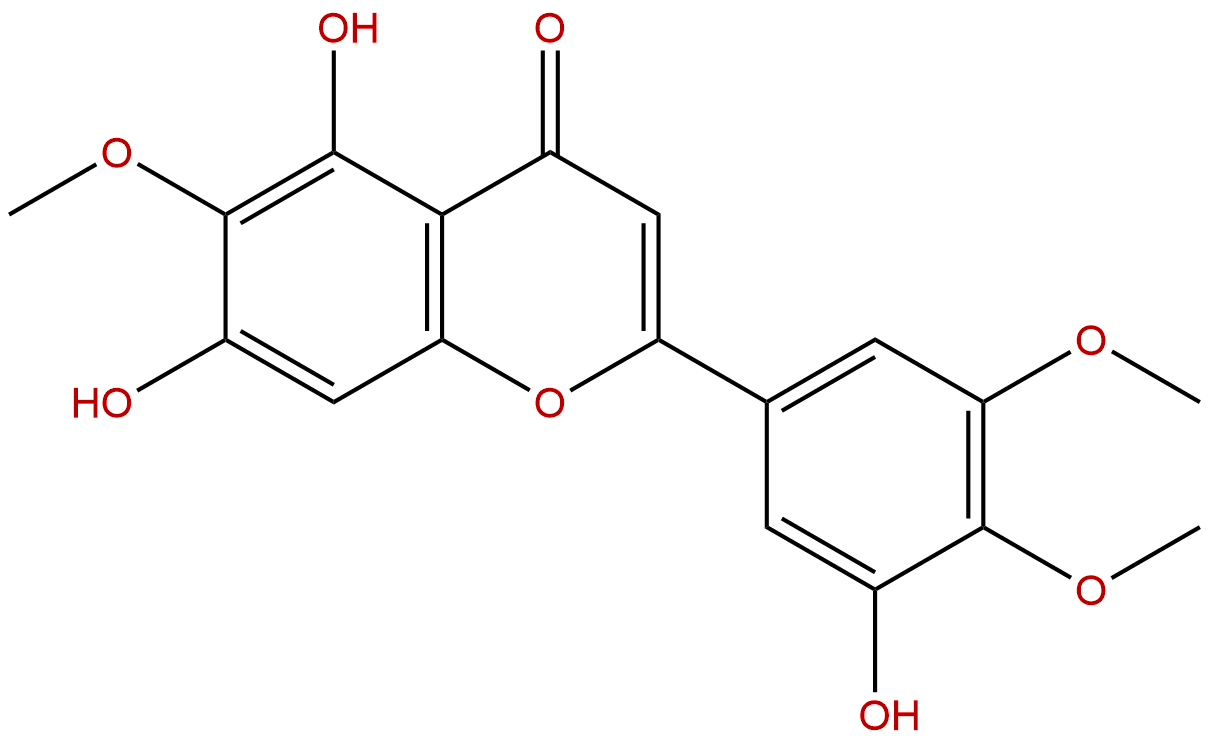
5,7,3'-Trihydroxy-6,4',5'-trimethoxyflavoneCAS No.:78417-26-2 |
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1766 |
| Formula: | C18H16O8 |
| Mol Weight: | 360.318 |
| Botanical Source: | Aurantii fructus immaturus |
Product name: 5,7,3'-Trihydroxy-6,4',5'-trimethoxyflavone
Synonym name:
Catalogue No.: BP1766
Cas No.: 78417-26-2
Formula: C18H16O8
Mol Weight: 360.318
Botanical Source: Eupatorium capillifolium; Artemisia argyi
Physical Description:
Type of Compound: Flavonoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
The product could be supplied from milligrams to grams. Inquire for bulk scale.
We provide solution to improve the water-solubility of compounds, thereby facilitating the variety of activity tests and clinic uses.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
5,7,3'-Trihydroxy-6,4',5'-trimethoxyflavone is a natural product from Artemisia argyi.
References:
Nat Prod Commun. 2014 Feb;9(2):163-4.
Further characterization of foliar flavonoids in Crossostephium chinense and their geographic variation.
METHODS AND RESULTS:
Foliar flavonoids of Crossostephium chinense in Japan and Taiwan were isolated and further characterized. Eighteen flavonoid aglycones, luteolin, apigenin, hispidulin, chrysoeriol, 5,7,4'-trihydroxy-6,3',5'-trimethoxyflavone, jaceosidin, cilsimaritin, quercetin 3-methyl ether, axillarin, chrysosplenol-D, cirsiliol, apometzgerin, 5,7,3'-Trihydroxy-6,4',5'-trimethoxyflavone, luteolin 3',4'-dimethyl ether, cirsilineol, eupatilin, nepetin and 5,7,3',4'-tetrahydroxy-6,5'-dimethoxyflavone, were identified by UV, 1H and 13C NMR spectroscopic, LC-MS and HPLC comparisons w ith authentic samples. The compounds existed on the leaf surface. Four flavonoid glycosides, quercetin 3,7-di-O-glucoside, quercetin 3-O-rutinoside, luteolin 7-O-glucoside and apigenin 7-O-rutinoside, were also isolated as the intracellular flavonoids.
CONCLUSIONS:
It was shown by HPLC survey that variation of the species' flavonoids occurs among the collection sites.