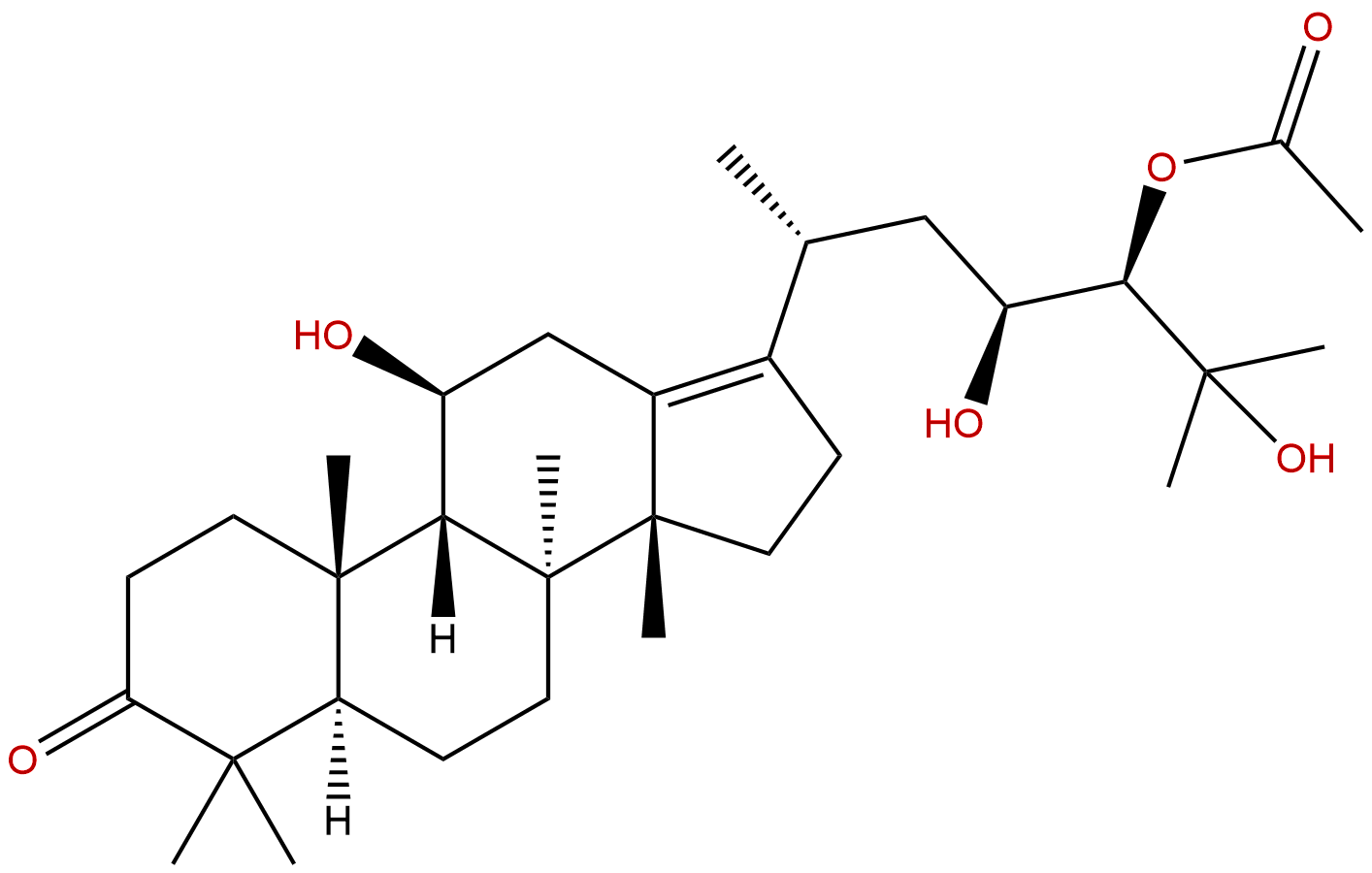
Alisol A,24-acetateCAS No.:18674-16-3 |
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0138 |
| Formula: | C32H52O6 |
| Mol Weight: | 532.762 |
| Botanical Source: | Alisma orientale(Sam.) Juzep. |
Product name: Alisol A,24-acetate
Synonym name:
Catalogue No.: BP0138
Cas No.: 18674-16-3
Formula: C32H52O6
Mol Weight: 532.762
Botanical Source: Alisma orientale(Sam.) Juzep.
Physical Description:
Type of Compound: Triterpenoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Alisol A 24-acetate has antibacterial activity, it also has anti-complement activity against the classical pathway of the complement system with IC50 values of 130 microM. Alisol A 24-acetate can effectively prevent bone loss in ovariectomized (OVX) mice, and that it can be considered a potential therapeutic for the treatment of postmenopausal osteoporosis.
References:
Anal Bioanal Chem. 2011 Jan;399(3):1363-9.
A sensitive liquid chromatography-mass spectrometry method for simultaneous determination of alisol A and alisol A 24-acetate from Alisma orientale (Sam.) Juz. in rat plasma.
METHODS AND RESULTS:
A liquid chromatography-mass spectrometry (LC-MS) method was developed and validated for the simultaneous determination of alisol A and Alisol A 24-acetate from Alisma orientale (Sam.) Juz. in rat plasma using diazepam as an internal standard. A 200-μl plasma sample was extracted by methyl tert-butyl ether and the separation was performed on Kromasil C(18) column (150 × 4.6 mm, 5 μm) with the mobile phase of acetonitrile (containing 0.1% of formic acid)-water (73:27, v/v) at a flow rate of 0.8 ml/min in a run time of 10 min. The two analytes were monitored with positive electrospray ionization by selected ion monitoring mode. The lower limit of quantitation for both alisol A and Alisol A 24-acetate were 10 ng/ml. The calibration curves were linear in the measured range 10-1,000 ng/ml for alisol A and 10-500 ng/ml for Alisol A 24-acetate. The mean extraction recoveries were above 74.7% for alisol A and above 72.4% for Alisol A 24-acetate from biological matrixes. The intra- and inter-day precision for all concentrations of quality controls was lower than 14.1% (RSD %) for each analyte. The accuracy ranged from -12.3% to 9.8% (RE %) for alisol A, and -8.6% to 14.2% (RE %) for Alisol A 24-acetate.
CONCLUSIONS:
The method was successfully applied to the study on the pharmacokinetics of alisol A and Alisol A 24-acetate in rat plasma.
Arch Pharm Res. 2003 Jun;26(6):463-5.
Anti-complementary activity of protostane-type triterpenes from Alismatis rhizoma.
Four protostane-type triterpenes, alisol B 23-acetate (1a), alisol C 23-acetate (2a), alisol B (3a), and Alisol A 24-acetate (4a), were isolated from the rhizome of Alismatis plantago-aquatice L. var. orientale Samuelson (Alismataceae) and eleven protostane derivatives (compounds 1-11) were obtained by selective modification from alisol B 23-acetate (1a).
METHODS AND RESULTS:
These compounds were investigated for their anti-complement activity against the classical pathway of the complement system. Alisol B (3a) and Alisol A 24-acetate (4a) exhibited anti-complement activity with IC50 values of 150 and 130 microM. Among the synthetic derivatives, the tetrahydroxylated protostane triterpene (9) showed moderate inhibitory activity with IC50 value of 97.1 microM.
CONCLUSIONS:
Introduction of an aldehyde group at C-23 (10; IC50 value, 47.7 microM) showed the most potent inhibitory effect on the complement system in vitro.
Arch Pharm Res. 2012 Nov;35(11):1919-26.
A new triterpenoid from Alisma orientale and their antibacterial effect.
A new triterpenoid, named alisol Q 23-acetate, as well as fourteen known terpenes, alisol B 23-acetate (2), alisol B (3), alismol (4), 10-O-methyl-alismoxide (5), alismoxide (6), 11-deoxyalisol C (7), 13β,17β-epoxyalisol B 23-acetate (8), 4β,12-dihydroxyguaian-6,10-diene (9), alisol C 23-acetate (10), alisolide (11), 16β-methoxyalisol B monoacetate (12), alisol A (13), 16β-hydroxyalisol B 23-acetate (14), Alisol A 24-acetate (15) were isolated from the rhizomes of Alisma orientale. The structures of compounds (1-15) were identified based on 1D and 2D NMR, including (1)H-(1)H COSY, HSQC, HMBC and NOESY spectroscopic analyses.
METHODS AND RESULTS:
Among these isolates, antibacterial effect of compounds 2, 3, 10, and 15, major constituents of A. orientale was examined. The MIC values of compounds 2, 10, and 15 were 5-10 βg/mL against eight antibiotic resistant strains, which were lower than those from the positive controls (MICs of chloramphenicol and ampicillin were 5-80 μg/mL).
CONCLUSIONS:
Therefore, compounds 2, 10 and 15 exhibited the potent antibacterial activity.
Molecules. 2016 Jan 9;21(1):74.
The Protective Effects of Alisol A 24-Acetate from Alisma canaliculatum on Ovariectomy Induced Bone Loss in Vivo.
Alisma canaliculatum is a herb commonly used in traditional Korean medicine, and has been shown in scientific studies to have antitumor, diuretic hepatoprotective, and antibacterial effects. Recently, the anti-osteoclastogenesis of Alisol A 24-acetate from Alisma canaliculatum was investigated in vitro. However, the influence of Alisol A 24-acetate on osteoporosis in animals has not been investigated.
METHODS AND RESULTS:
The present study was undertaken to investigate the anti-osteoporotic effect of Alisol A 24-acetate on bone mass in ovariectomized (OVX) mice and to identify the mechanism responsible for its effects. OVX mice were treated daily with 0.5 or 2 μg/g of Alisol A 24-acetate for a period of six weeks. It was found that these administrations significantly suppressed osteoporosis in OVX mice and improved bone morphometric parameters. The serum estradiol, bone alkaline phosphatase levels, regulatory T/Th17 cell numbers were significantly increased by Alisol A 24-acetate as compared with untreated OVX mice. In addition, TRAP activity was inhibited by Alisol A 24-acetate in OVX mice.
CONCLUSIONS:
These results suggest Alisol A 24-acetate effectively prevents bone loss in OVX mice, and that it can be considered a potential therapeutic for the treatment of postmenopausal osteoporosis.
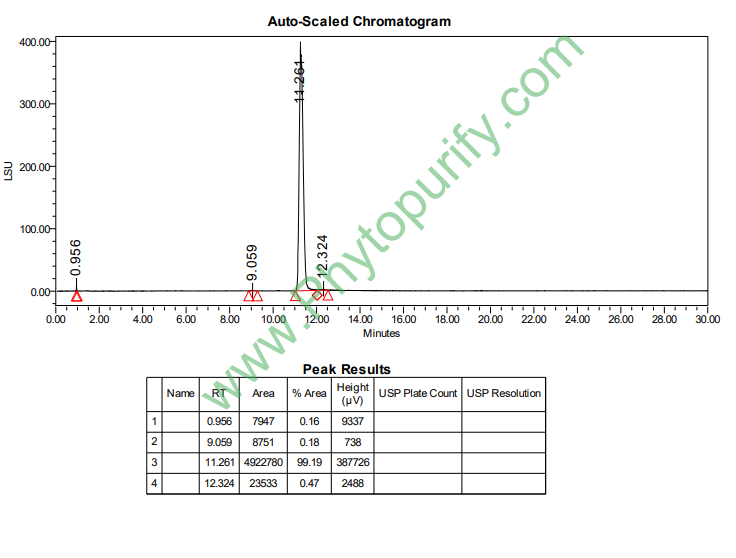
HPLC of 24-acetate