
DehydrocorydalinCAS No.:30045-16-0 |
||||||||||
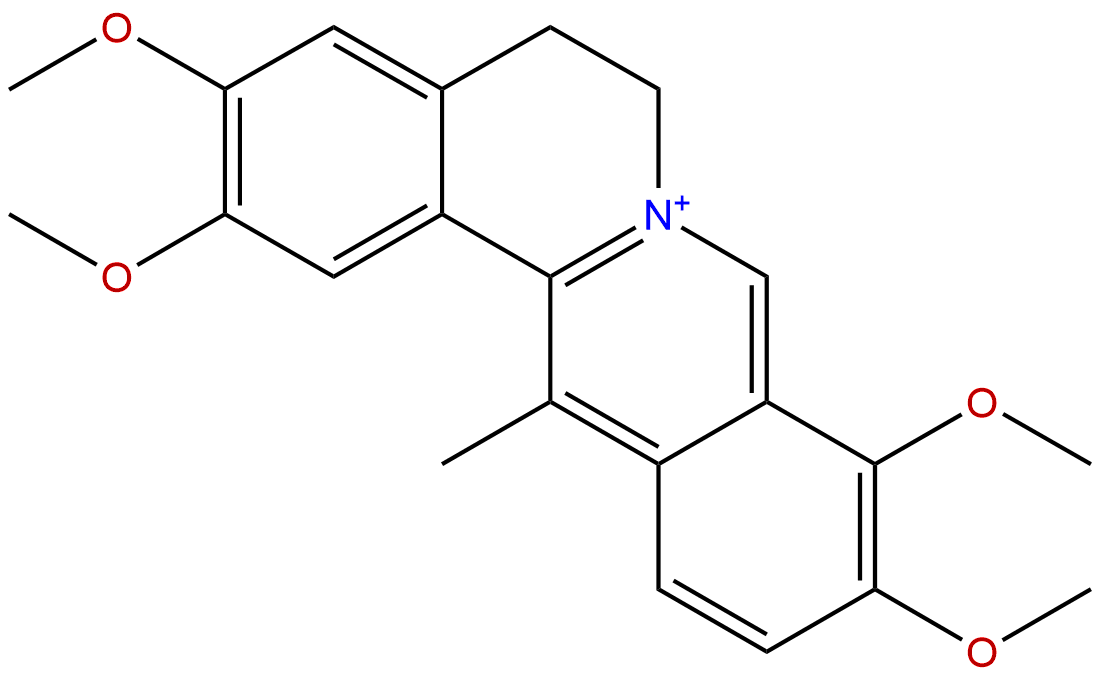 |
|
|
||||||||

| Catalogue No.: | BP0460 |
| Formula: | C22H24NO4+ |
| Mol Weight: | 366.436 |
| Botanical Source: | Corydalis yanhusuo W.T.Wang |
Synonym name:
Catalogue No.: BP0460
Cas No.: 30045-16-0
Formula: C22H24NO4+
Mol Weight: 366.436
Botanical Source: Quaternary alkaloid from Corydalis ambigua and several other Corydalis spp. and from Berberis floribunda (Fumariaceae, Berberidaceae)
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Description:
Dehydrocorydalin has anti-inflammatory,antinociceptive,antiplatelet,and anti-tumor effects and can protect the cardiovascular system. Dehydrocorydaline stimulates p38 MAPK activation, which can enhance heterodimerization of MyoD and E proteins, thus resulting in MyoD activation and myoblast differentiation. Dehydrocorydaline inhibits MCF-7 cell proliferation by inducing apoptosis mediated by regulating Bax/Bcl-2, activating caspases as well as cleaving PARP.
References:
Phytother Res. 2017 Mar;31(3):441-448.
Anti-Metastatic Effect of Dehydrocorydaline on H1299 Non-Small Cell Lung Carcinoma Cells via Inhibition of Matrix Metalloproteinases and B Cell Lymphoma 2.
Though Dehydrocorydaline, an alkaloid isolated from Corydalis turtschaninovii tuber, was known to have anti-coronary artery disease, anti-inflammatory, apoptotic, anti-allergic, anti-acetylcholinesterase, and antitumor effects, the underlying anti-metastatic mechanism of Dehydrocorydalin was never elucidated in lung cancer cells so far.
METHODS AND RESULTS:
Thus, in the present study, the anti-metastatic effect of Dehydrocorydaline was examined in non-small cell lung carcinoma (NSCLC) cells, mainly targeting matrix metalloproteinases (MMPs) and B cell lymphoma-2 (Bcl-2) signaling. Here, Dehydrocorydaline exerted weak cytotoxicity and attenuated the protein expression of Bcl-2 and activated Bax in a concentration-dependent manner in NSCLC cells, such as A549, H460, H1299, and H596 cells. Also, Dehydrocorydaline suppressed the migration of H1299 cells by wound healing assay and transwell migration assay. Consistently, Dehydrocorydaline attenuated mRNA and protein levels of MMP7 and MMP9 as metastasis biomarkers in H1299 cells by quantitative reverse transcription polymerase chain reaction. Of note, Bcl-2 overexpression reduced the cytotoxic and anti-metastatic effects of Dehydrocorydaline on pCDNA-Bcl-2 transfected H1299 cells.
CONCLUSIONS:
Overall, our findings provide scientific evidence that Dehydrocorydaline exerts anti-metastatic potential via suppression of MMPs and Bcl-2 signaling in NSCLC cells.
Mol Med Rep. 2016 Oct;14(4):3029-36.
Dehydrocorydaline promotes myogenic differentiation via p38 MAPK activation.
Muscle regeneration is a coordinated process that involves proliferation and differentiation of muscle progenitor cells. Activation of MyoD is a key event in myogenic differentiation, which is regulated by p38 mitogen‑activated protein kinases (MAPK). In a screen of natural compounds for the enhancement of MyoD activity, Dehydrocorydaline (DHC) from the Corydalis tuber was identified.
METHODS AND RESULTS:
Treatment of C2C12 myoblasts with DHC increased the expression levels of muscle‑specific proteins, including MyoD, myogenin and myosin heavy chain. In addition, C2C12 myoblasts exhibited enhanced multinucleated myotube formation without any cytotoxicity. Treatment with DHC elevated p38 MAPK activation and the interaction of MyoD with an E protein, which is likely to result in activation of MyoD and promotion of myoblast differentiation. Furthermore, defects in differentiation‑induced p38 MAPK activation and myoblast differentiation induced by depletion of the promyogenic receptor protein Cdo in C2C12 myoblasts were restored by DHC treatment.
CONCLUSIONS:
In conclusion, these results indicated that DHC stimulates p38 MAPK activation, which can enhance heterodimerization of MyoD and E proteins, thus resulting in MyoD activation and myoblast differentiation. These findings suggested that DHC may be considered a potential therapeutic compound for the improvement of muscle stem cell regenerative capacity in injured muscle.
Sci Rep. 2016 Jun 7;6:27129.
Antinociceptive effects of dehydrocorydaline in mouse models of inflammatory pain involve the opioid receptor and inflammatory cytokines.
Dehydrocorydaline (DHC) is an alkaloidal component isolated from Rhizoma corydalis. Previous studies have shown that DHC has anti-inflammatory and anti-tumor effects and that it can protect the cardiovascular system. However, there are few studies of the antinociceptive effects of DHC in vivo.
METHODS AND RESULTS:
This study explored the antinociceptive effects and possible mechanisms of DHC in mice using two inflammatory pain models: the acetic acid-induced writhing test and the formalin paw test. The intraperitoneal administration of DHC (3.6, 6 or 10 mg/kg) showed a dose-dependent antinociceptive effect in the acetic acid-induced writhing test and significantly attenuated the formalin-induced pain responses in mice. The antinociceptive effects of DHC were not associated with changes in the locomotor activity or motor responses of animals, and no obvious acute or chronic toxic effects were observed in the mice. Furthermore, the use of naloxone confirmed the involvement of the opioid receptor in the central antinociceptive effects of DHC. DHC reduced formalin-induced paw edema, which indicated that DHC may produce an anti-inflammatory effect in the periphery. In the formalin test, DHC decreased the expression of caspase 6 (CASP6), TNF-α, IL-1β and IL-6 proteins in the spinal cord.
CONCLUSIONS:
These findings confirm that DHC has antinociceptive effects in mice.