IsorhamnetinCAS No.:480-19-3 |
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0794 |
| Formula: | C16H12O7 |
| Mol Weight: | 316.265 |
| Botanical Source: | Hippophae rhamnoides L. |
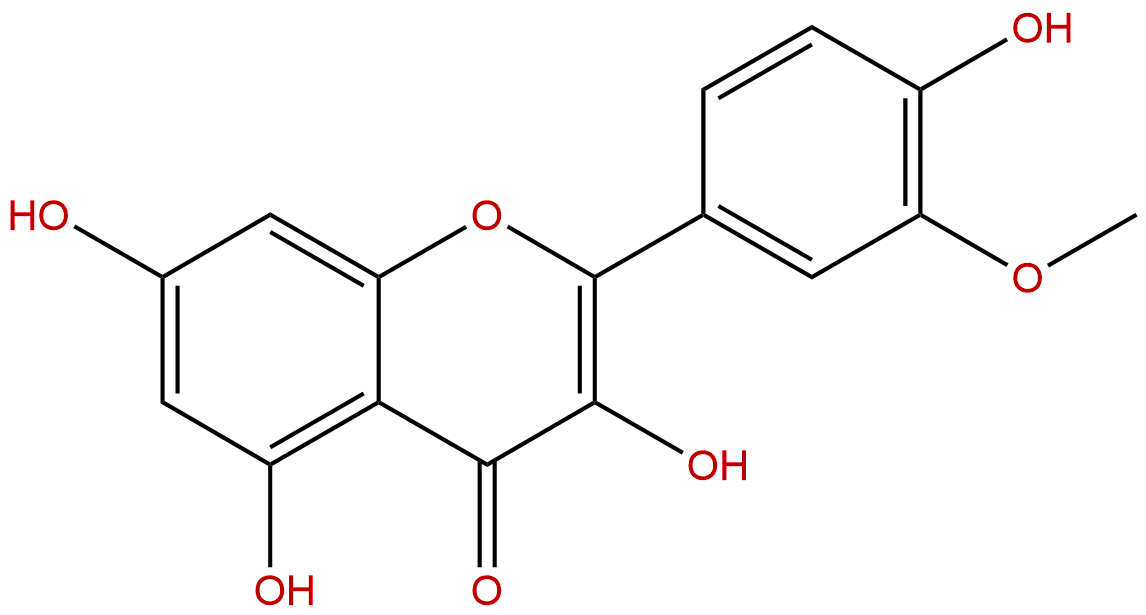
Product name: Isorhamnetin
Synonym name: Isorhamnetol; Quercetin 3′-methyl ether
Catalogue No.: BP0794
Cas No.: 480-19-3
Formula: C16H12O7
Mol Weight: 316.265
Botanical Source: Hippophae rhamnoides L.
Physical Description:
Type of Compound: Flavonoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Isorhamnetin, a natural flavonol aglycon, is a tyrosinase inhibitor and has anti-adipogenic, cardioprotective, anti-tumor, and antioxidant activities. it inhibits the H(2)O(2)-induced activation of the intrinsic apoptotic pathway via ROS scavenging and ERK inactivation, it inhibits NF-κB signaling. Isorhamnetin prevents angiotensin II (AngII)-induced endothelial dysfunction by inhibiting the overexpression of p47(phox) and the subsequent increases O2-production, resulting in increased nitric oxide bioavailability.
References:
J Periodontal Res. 2013 Dec;48(6):687-95.
Isorhamnetin inhibits Prevotella intermedia lipopolysaccharide-induced production of interleukin-6 in murine macrophages via anti-inflammatory heme oxygenase-1 induction and inhibition of nuclear factor-κB and signal transducer and activator of transcrip
Interleukin-6 (IL-6) is a key proinflammatory cytokine that has been considered to be important in the pathogenesis of periodontal disease. Therefore, host-modulatory agents directed at inhibiting IL-6 appear to be beneficial in terms of attenuating periodontal disease progression and potentially improving disease susceptibility. In the current study, we investigated the effect of the flavonoid Isorhamnetin on the production of IL-6 in murine macrophages stimulated with lipopolysaccharide (LPS) from Prevotella intermedia, a pathogen implicated in inflammatory periodontal disease, and its mechanisms of action.
METHODS AND RESULTS:
Lipopolysaccharide from P. intermedia ATCC 25611 was isolated using the standard hot phenol-water method. Culture supernatants were collected and assayed for IL-6. We used real-time PCR to quantify IL-6 and heme oxygenase-1 (HO-1) mRNA expression. The expression of HO-1 protein and the levels of signaling proteins were monitored using immunoblot analyses. The DNA-binding activity of nuclear factor-κB (NF-κB) was analyzed using ELISA-based assay kits. Isorhamnetin significantly down-regulated P. intermedia LPS-induced production of IL-6 as well as its mRNA expression in RAW264.7 cells. Isorhamnetin up-regulated the expression of HO-1 at both gene transcription and translation levels in cells stimulated with P. intermedia LPS. In addition, inhibition of HO-1 activity by tin protoporphyrin IX blocked the inhibitory effect of Isorhamnetin on IL-6 production. Isorhamnetin failed to prevent LPS from activating either c-Jun N-terminal kinase or p38 pathways. Isorhamnetin did not inhibit NF-κB transcriptional activity at the level of inhibitory κB-α degradation. Isorhamnetin suppressed NF-κB signaling through inhibition of nuclear translocation and DNA binding activity of NF-κB p50 subunit and attenuated signal transducer and activator of transcription 1 signaling.
CONCLUSIONS:
Although further research is required to clarify the detailed mechanism of action, we propose that Isorhamnetin may contribute to blockade of the host-destructive processes mediated by IL-6 and could be a highly efficient modulator of the host response in the treatment of inflammatory periodontal disease. Further research in animal models of periodontitis is required to better evaluate, the potential of Isorhamnetin as a novel agent for treating periodontal disease.
J Nutr. 2007 Apr;137(4):910-5.
Quercetin and isorhamnetin prevent endothelial dysfunction, superoxide production, and overexpression of p47phox induced by angiotensin II in rat aorta.
The dietary flavonoid quercetin reduces blood pressure and improves endothelial function in several rat models of hypertension.
METHODS AND RESULTS:
We analyzed the effects of quercetin and its methylated metabolite Isorhamnetin on the aortic endothelial dysfunction induced by incubation with angiotensin II (AngII) in vitro for 6 h. AngII diminished the relaxant responses to acetylcholine in phenylephrine-contracted aorta. Coincubation with quercetin or Isorhamnetin, or addition of superoxide (O(2)(-)) dismutase or apocynin to the assay medium, prevented these inhibitory effects. At 6 h, AngII induced a marked increase in O(2)(-) production as measured by dihydroethidium fluorescence, which was prevented by quercetin and Isorhamnetin. AngII also increased the expression of p47(phox), a regulatory subunit of the membrane NADPH oxidase. Immunohistochemical analysis revealed that overexpression of p47(phox) occurred mainly in the medial layer. p47(phox) overexpression was also prevented by quercetin and Isorhamnetin.
CONCLUSIONS:
Taken together, these results show for the first time, to our knowledge, that quercetin and Isorhamnetin prevent AngII-induced endothelial dysfunction by inhibiting the overexpression of p47(phox) and the subsequent increased O(2)(-) production, resulting in increased nitric oxide bioavailability.
Life Sci. 2010 Mar 13;86(11-12):416-23.
Isorhamnetin-induced anti-adipogenesis is mediated by stabilization of beta-catenin protein.
Previous studies have shown that Isorhamnetin has anti-adipogenic effects in mouse 3T3-L1 cells. This study was conducted to elucidate the inhibitory mechanisms of Isorhamnetin during adipogenic differentiation of human adipose tissue-derived stem cells (hAMSCs).
METHODS AND RESULTS:
The effect of Isorhamnetin on adipogenic differentiation of hAMSCs was quantified by Oil Red O staining and a triglyceride assay. In addition, real-time PCR and Western blot were used to determine the expression of adipogenesis-related genes. Isorhamnetin inhibited the adipocyte differentiation of hAMSCs. Additionally, when the effects of Wnt antagonists that promote adipogenesis were evaluated, Isorhamnetin was found to down-regulate the mRNA levels of sFRP1 and Dkk1, but had no effect on the mRNA levels of sFRP2, sFRP3, sFRP4 and Dkk3. Isorhamnetin also inhibited the expression of Wnt receptor and co-receptor genes. Furthermore, Isorhamnetin increased the protein levels of beta-catenin, an effector molecule of Wnt signaling, but had no effect on the mRNA levels of beta-catenin. The phosphorylation level of GSK 3beta was also increased by Isorhamnetin. These results were confirmed by the fact that the expression of c-myc, cyclin D1 and PPARdelta, which are target genes of beta-catenin, was upregulated by Isorhamnetin. Moreover, Isorhamnetin reduced the mRNA expression levels of C/EBPalpha and PPARgamma, which are known to be inhibited by c-myc or by cyclin D1 and PPARdelta, respectively.
CONCLUSIONS:
Our results indicate that Isorhamnetin inhibits the adipogenic differentiation of hAMSCs and that its mechanisms are mediated by the stabilization of beta-catenin.
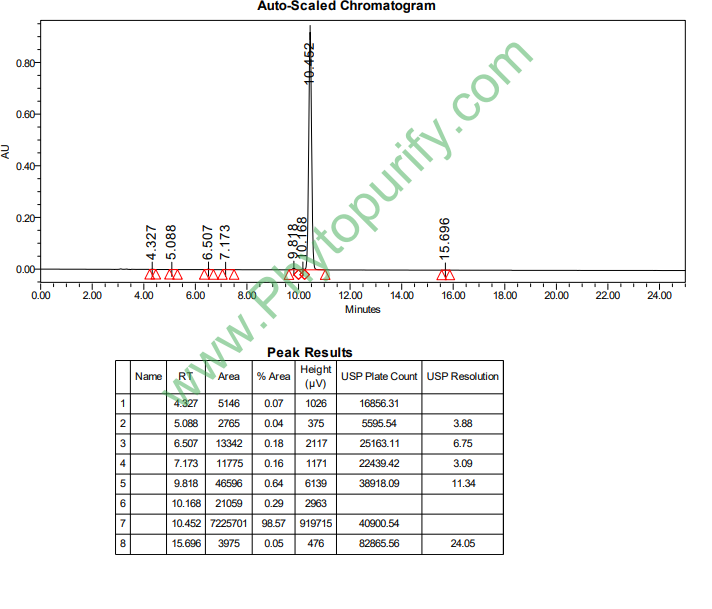
HPLC of Isorhamnetin
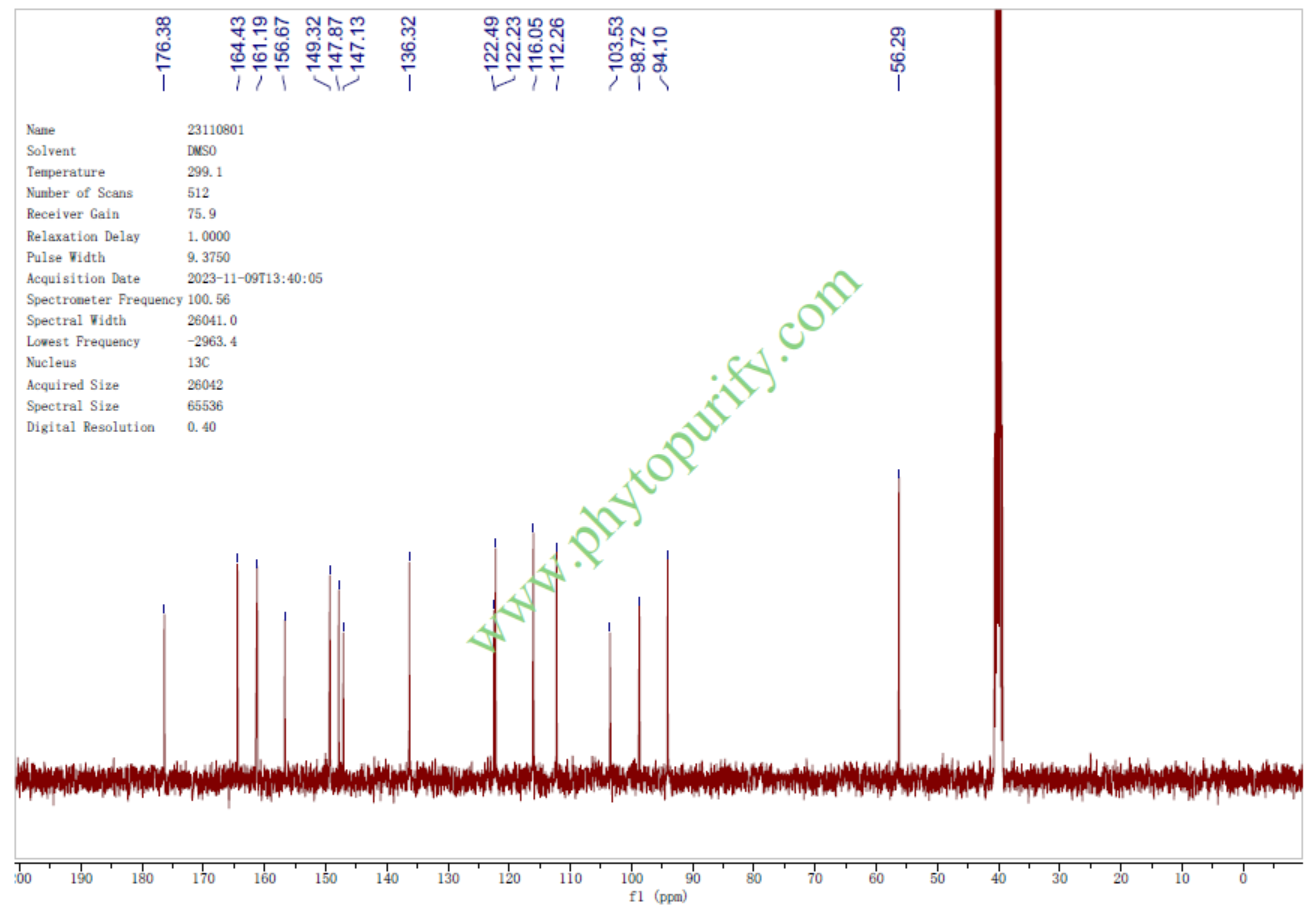
NMR of Isorhamnetin