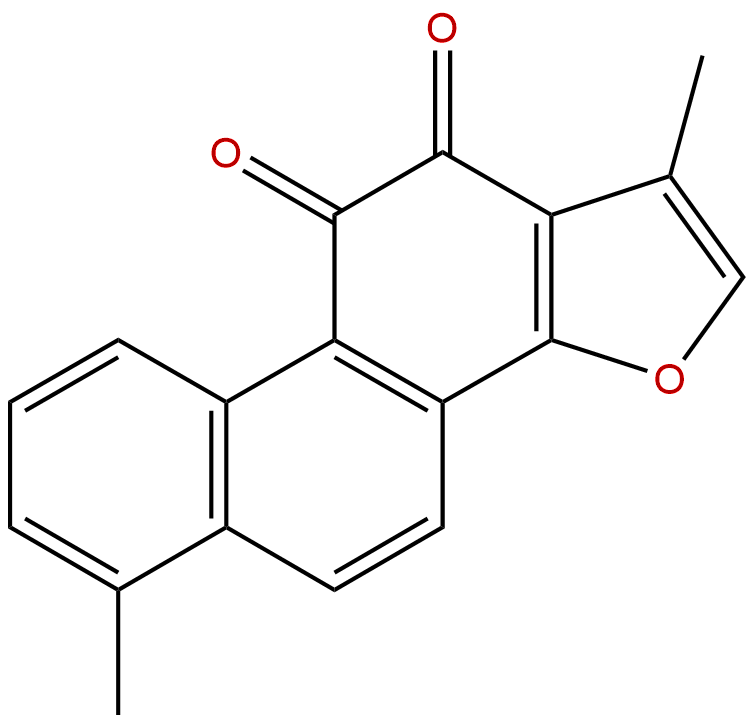
Product name: Tanshinone I
Synonym name: Tanshinquinone I
Catalogue No.: BP1361
Cas No.: 568-73-0
Formula: C18H12O3
Mol Weight: 276.291
Botanical Source: Pigment from Root of several Salvia spp.
Physical Description: Red powder
Type of Compound: Diterpenoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams, up to kilograms
Inquire for bulk scale.
Descriptions:
Tanshinone I, a major active principle of Salvia miltiorrhiza (Danshen), has been shown to overcome tumor drug resistance and metastasis, it inhibits angiogenesis, inhibits proliferation, migration and tube formation of vascular endothelial cells, rat aortic ring sprouting and the neovascularization of the chick chorioallantoic membrane in a concentration-dependent manner; it as promising angiogenesis inhibitors.[1]
Tanshinone I can suppress growth and invasion of human breast cancer cells, MDA-MB-231, through regulation of adhesion molecules.[2]
Tanshinone I, tanshinone IIA and cryptotanshinone, can protect against liver toxicity in vivo and in vitro due to its antioxidant effects.[3]
Tanshinone I exerts neuroprotection in cerebrovascular diseases including transient ischemic attack, it can promote neurogenesis in the mouse DG and that the neurogenesis is related with increases of Wnt-3, p-GSK-3β and β-catenin immunoreactivities.[4]
Tanshinone I can effectively induce apoptosis in estrogen receptor-positive (MCF-7) and estrogen receptor-negative (MDA-MB-231) breast cancer cells; it has growth inhibition and apoptosis induction in human colon cancer Colo 205 cells.[5]
Tanshinone I enhances learning and memory, and ameliorates memory impairment in mice via the extracellular signal-regulated kinase signalling pathway.[6,7]
References:
[1] Wang Y, Li J X, Wang Y Q, et al. Oncotarget, 2015, 6(18):16031-42.
[2] Nizamutdinova I T, Lee G W, Lee J S, et al. Carcinogenesis, 2008, 29(10):1885-92.
[3] Park E J, Zhao Y Z, Kim Y C, et al. Food Chem Toxicol , 2009, 47(11):2742-8.
[4] Bai H C, Park J H, Cho J H, et al. Neurochem Res, 2016,41(8):1958-68.
[5] Nizamutdinova IT, Lee GW, Son KH, et al. Int J Oncol, 2008, 33(3):485-91.
[5] Su C C, Chen G W, Lin J G. Int J Mol Med, 2008, 22(5):613-8.
[6] Dong H K, Kim S, Su J J, et al. Brit J Pharmacol, 2009, 158(4):1131-42.
[7] Shi Z, He J, Yao T, et al. J Pharm Biomed Anal, 2005, 37(3):481-6.