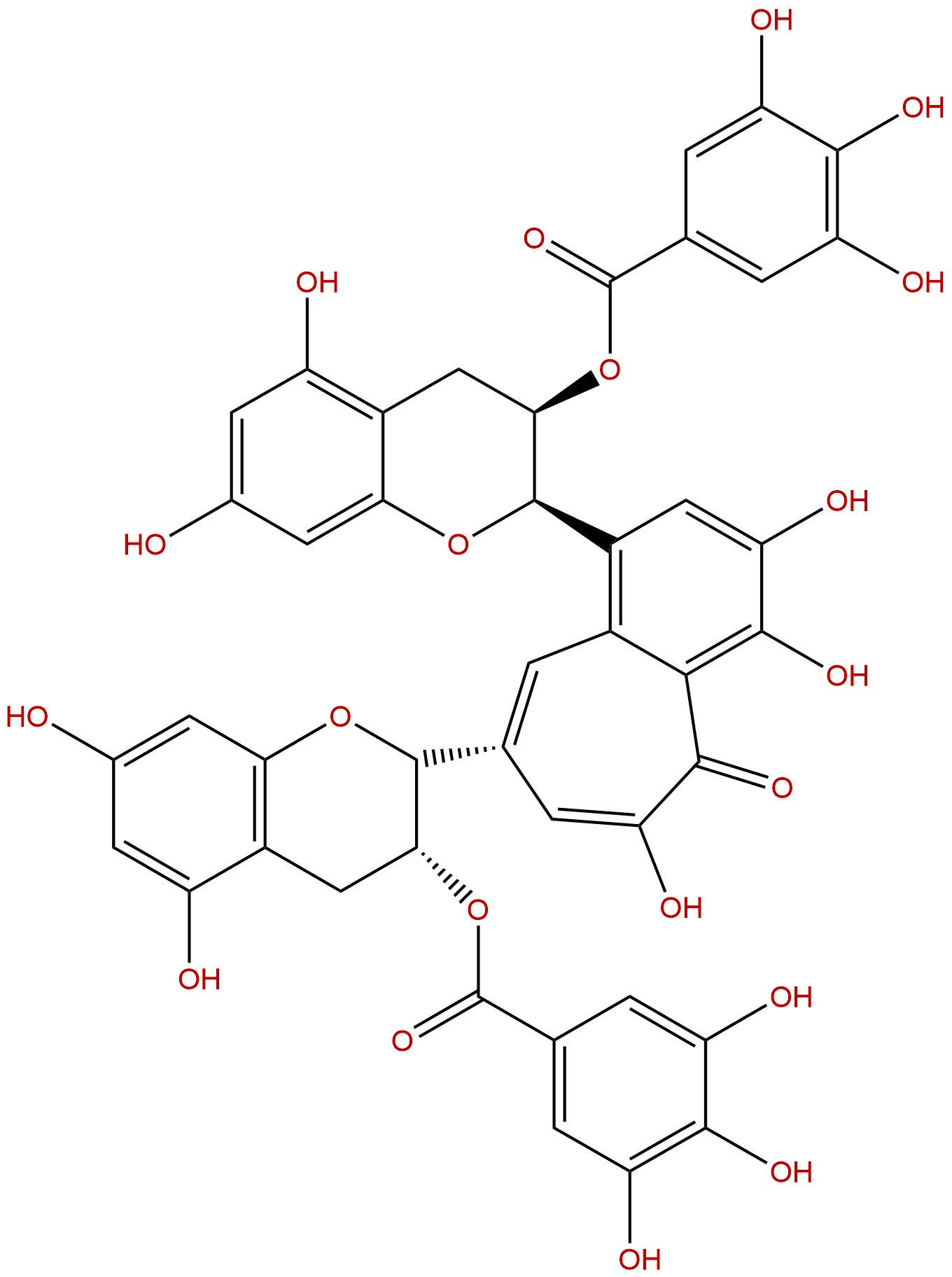
Theaflavin 3,3'-digallateCAS No.:30462-35-2 |
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1385 |
| Formula: | C43H32O20 |
| Mol Weight: | 868.709 |
| Botanical Source: | Black Tea |
Product name: Theaflavine-3,3'-digallate
Synonym name: Theaflavine bisgallate; Teaflavin digallate; Theaflavine-3,3'-digallate; Other Cas, 33377-72-9
Catalogue No.: BP1385
Cas No.: 30462-35-2
Formula: C43H32O20
Mol Weight: 868.709
Botanical Source: Black Tea
Physical Description:
Type of Compound: Polyphenols
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Theaflavin-3,3'-digallate(TF3), an inducer of oxidative stress, which has anti-inflammatory and cancer chemopreventive actions, it reduces tumor angiogenesis by downregulating HIF-1αand VEGF; suggests that TF3 might serve as a potential anti-angiogenic agent for cancer treatment. TF3 and lactic acid combinations can reduce Herpes Simplex Virus(HSV) infectivity.
References:
J Agric Food Chem. 2009 Jul 8;57(13):5816-22.
Increase of theaflavin gallates and thearubigins by acceleration of catechin oxidation in a new fermented tea product obtained by the tea-rolling processing of loquat ( Eriobotrya japonica ) and green tea leaves.
In a project to produce a new fermented tea product from non-used tea leaves harvested in the summer, we found that kneading tea leaves ( Camellia sinensis ) with fresh loquat leaves ( Eriobotrya japonica ) accelerated the enzymatic oxidation of tea catechins.
METHODS AND RESULTS:
The fermented tea obtained by tea-rolling processing of tea and loquat leaves had a strong, distinctive flavor and a plain aftertaste, which differed from usual black, green, and oolong teas. The phenolic constituents were similar to those of black tea. However, the concentrations of theaflavin 3-O-gallate, Theaflavin 3,3'-di-O-gallate, and thearubigins were higher in the tea leaves kneaded with loquat leaves than in tea leaves kneaded without loquat leaves.
CONCLUSIONS:
The results from in vitro experiments suggested that acceleration of catechin oxidation was caused by the strong oxidation activity of loquat leaf enzymes and a coupled oxidation mechanism with caffeoyl quinic acids, which are the major phenolic constituents of loquat leaves.
Biosci Biotechnol Biochem. 2003 Feb;67(2):396-401.
Evaluation of the anti-oxidative effect (in vitro) of tea polyphenols.
Forty-three polyphenols from tea leaves were evaluated for their anti-oxidative effect against lipid peroxidation by the ferric thiocyanate method in vitro.
METHODS AND RESULTS:
Among these, 1,4,6-tri-O-galloyl-beta-D-glucose (hydrolyzable tannin) showed the highest anti-oxidative activity against lipid peroxidation, even stronger than that of 3-tert.-butyl-4-hydroxyanisole (BHA). The assay demonstrates that tea polyphenols, except for desgalloylated dimeric proanthocyanidins that possess a catechin structure in the upper unit and desgalloylated flavan-3-ols, and excepting Theaflavin 3,3'-di-O-gallate, had more anti-oxidative activity than that of alpha-tocopherol.
CONCLUSIONS:
The chemical structure-activity relationship shows that the anti-oxidative action advanced with the condensation of two molecules of flavan-3-ols as well as with 3-O-acylation in the flavan skeleton such as that by galloyl, (3'-O-methyl)-galloyl, and p-coumaroyl groups.
Eur J Pharmacol. 1999 Feb 19;367(2-3):379-88.
Theaflavin-3,3'-digallate from black tea blocks the nitric oxide synthase by down-regulating the activation of NF-kappaB in macrophages.
METHODS AND RESULTS:
Electrophoretic mobility shift assay (EMSA) indicated that theaflavin-3,3'-digallate(Theaflavin 3,3'-di-O-gallate) blocked the activation of nuclear factor kappaB (NF-kappaB), a transcription factor necessary for inducible NO synthase induction. Theaflavin-3,3'-digallate(Theaflavin 3,3'-di-O-gallate) also blocked phosphorylation of IkappaB from cytosolic fraction and reduced lipopolysacchride-induced nuclear accumulation of transcription factor NF-kappaB p65 and p50 subunits.
CONCLUSIONS:
These results suggest that theaflavin-3,3'-digallate(Theaflavin 3,3'-di-O-gallate) decreases the protein levels of inducible NO synthase by reducing the expression of inducible NO synthase mRNA, and the reduction could be via preventing the activation of NF-kappaB, thereby inhibiting the induction of inducible NO synthase transcription. It was also demonstrated that the gallic acid moiety of theaflavin-3,3'-digallate(Theaflavin 3,3'-di-O-gallate) is essential for their potent anti-inflammation activity.
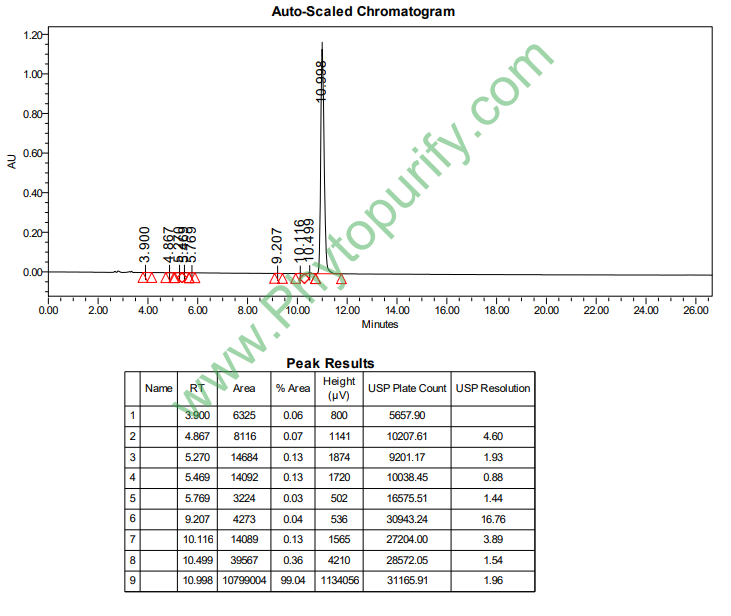
HPLC of Theaflavin digallate