
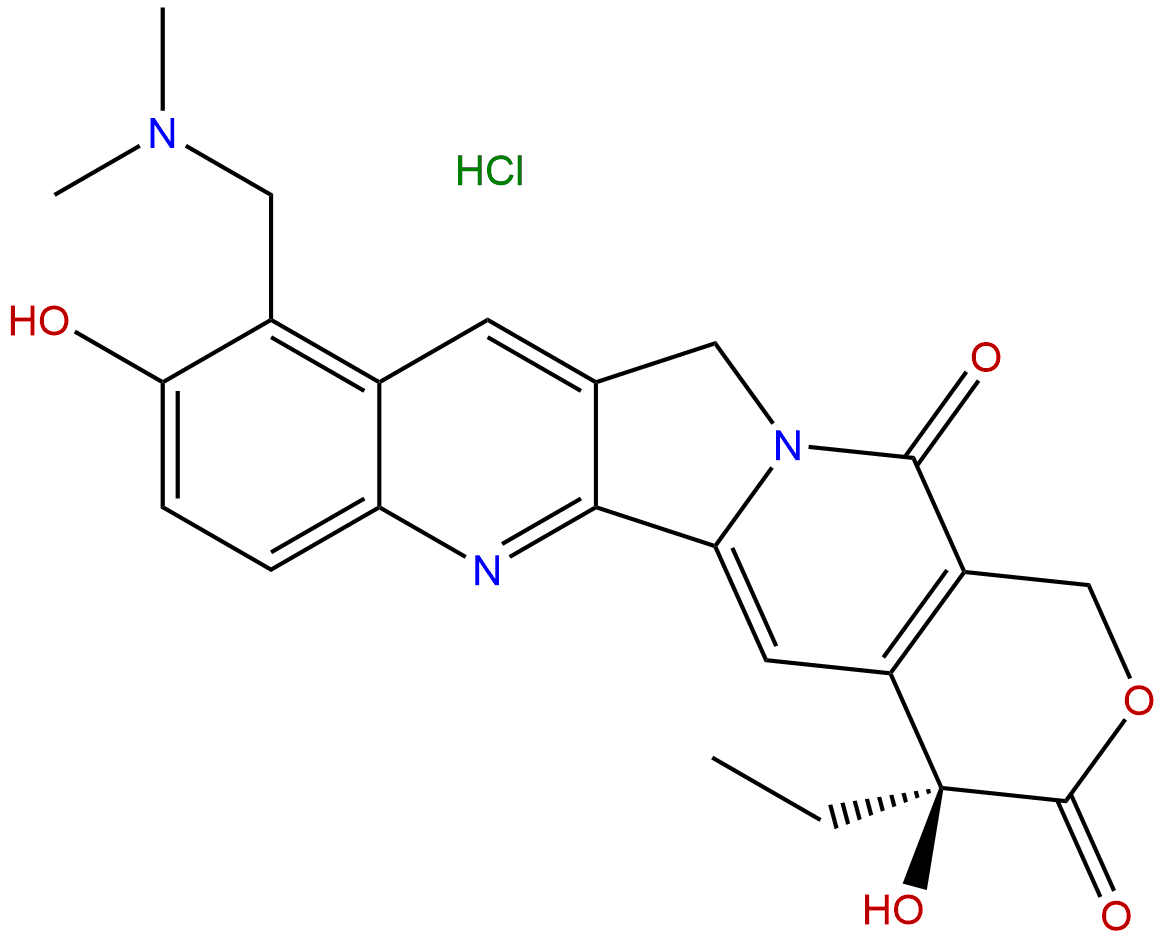
Topotecan HydrochlorideCAS No.:119413-54-6 |
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1399 |
| Formula: | C23H24ClN3O5 |
| Mol Weight: | 457.911 |
| Botanical Source: | Camptotheca acuminata. |
Synonym name:
Catalogue No.: BP1399
Cas No.: 119413-54-6
Formula: C23H24ClN3O5
Mol Weight: 457.911
Botanical Source:
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Description:
Topotecan hydrochloride, a topoisomerase I inhibitor, capable of inhibiting tumoral growth in animal models of retinoblastoma. Topotecan hydrochloride liposomes loaded CS/β-GP hydrogel could become a potential formulation for improving the antitumor efficacy of Topotecan hydrochloride.
References:
Histol Histopathol. 2012 Apr;27(4):497-506.
Topotecan hydrochloride effects on retinal vessels in newborn rats.
A physiological system, i.e. rodent retina during vessel formation and hierarchical organization, was utilised for assaying antiangiogenic properties of Topotecan, a topoisomerase I inhibitor, capable of inhibiting tumoral growth in animal models of retinoblastoma. In particular we analysed possible differences in effectiveness and side effects among different drug dosages and ways of administration.
METHODS AND RESULTS:
In the present research only qualitative analyses were undertaken. After preliminary experiments, in which suckling animals subcutaneously treated with Topotecan dosages comprised between 9 and 3 mg/kg underwent high lethality and extremely severe systemic damages, 7 day-old rats were subcutaneously, intravenously or peribulbary injected with a single dose of 1 mg/kg; retinal vessels were visualized in retinal fluorangio-graphies taken 1 and 2 weeks after treatment. The most important and frequent alterations were found to affect radial vessels, which showed non-perfused and/or regionally mislocated segments, together with abnormal branching and enlargements in retinal periphery; persistence of capillary-free periarteriolar regions, non-vascularised regions and spots of extravascular FITC were also detected. Despite the high individual variability the alterations were substantially similar among the different ways of drug administration, while they appeared milder in 21 day-old rats, with respect to younger ones.
CONCLUSIONS:
The extensive vascular remodelling found after Topotecan administration, besides demonstrating the antiangiogenic properties of this chemioterapic drug, confirms the rodent retina as a highly valuable model system for studying angiogenesis modulation.