
TrifolirhizinCAS No.:6807-83-6 |
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1406 |
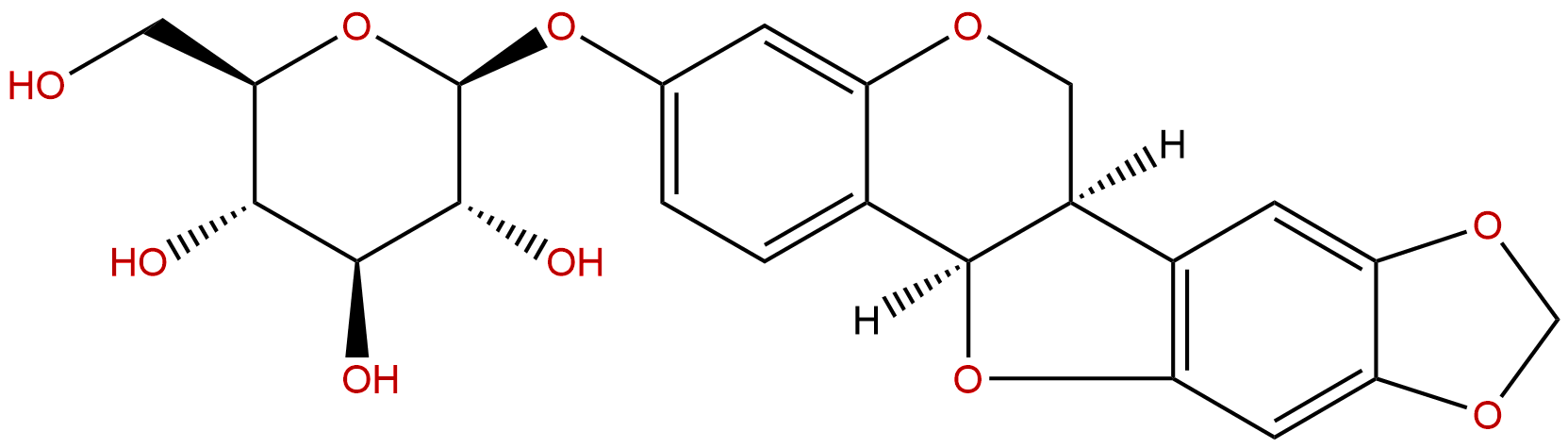
| Formula: | C22H22O10 |
| Mol Weight: | 446.408 |
| Botanical Source: | Trifolium pratense L. |
Product name: Trifolirhizin
Synonym name: Maackiain 3-glucoside
Catalogue No.: BP1406
Cas No.: 6807-83-6
Formula: C22H22O10
Mol Weight: 446.408
Botanical Source: Trifolium pratense L.
Physical Description:
Type of Compound: Flavonoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Trifolirhizin possesses potential anti-inflammatory and anti-cancer activities, it inhibits acetylcholine mediated airway smooth muscle (ASM) contraction or directly relaxes pre-contracted ASM independent of β 2 -adrenoceptors. Trifolirhizin may be a a candidate as skin-whitening agents, it exerts varying degrees of inhibition on tyrosinase-dependent melanin biosynthesis.
References:
J Agric Food Chem. 2009 Jun 10;57(11):4580-5.
Anti-Inflammatory and antiproliferative activities of trifolirhizin, a flavonoid from Sophora flavescens roots.
Trifolirhizin, a pterocarpan flavonoid, was isolated from the roots of Sophora flavescens, and its chemical structure was confirmed by (1)H and (13)C NMR and MS spectra.
METHODS AND RESULTS:
Its anti-inflammatory activity was examined in lipopolysaccharide (LPS)-stimulated mouse J774A.1 macrophages. Trifolirhizin not only dose-dependently inhibited LPS-induced expression of pro-inflammatory cytokines including tumor necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6) but also inhibited lipopolysaccharide (LPS)-induced expression of cyclooxygenase-2 (COX-2). In addition, Trifolirhizin showed in vitro inhibitory effects on the growth of human A2780 ovarian and H23 lung cancer cells.
CONCLUSIONS:
These results suggest that Trifolirhizin possesses potential anti-inflammatory and anticancer activities. Biol Pharm Bull. 2008 Jan;31(1):154-8.
Inhibitory effects of kurarinol, kuraridinol, and trifolirhizin from Sophora flavescens on tyrosinase and melanin synthesis.
Previously, it was reported that some prenylated flavonoids contained in the dichloromethane fraction of the ethanolic extract of Sophora flavescens, such as kuraridin, sophoraflavanone G, kurarinone, and kushenol F, are tyrosinase inhibitors; however, based on the level of these inhibitors in the extract, its inhibitory effect on tyrosinase activity was higher than expected. This has led us to further investigate other possible constituents that may contribute to the extract's strong inhibitory activity.
METHODS AND RESULTS:
The results of this study indicate that kurarinol (1), kuraridinol (2), and Trifolirhizin (3), from the ethyl acetate fraction of Sophora extract, can inhibit tyrosinase activity. Compared with kojic acid (16.22+/-1.71 microM), compounds 1-3 possessed potent tyrosinase inhibitory activity with IC(50) values of 8.60+/-0.51, 0.88+/-0.06, and 506.77+/-4.94 microM, respectively. These three compounds were further tested for their inhibitory effects on melanogenesis. In cultured B16 melanoma cells, 1-3 markedly inhibited (>50%) melanin synthesis at 50 microM.
CONCLUSIONS:
This is the first study indicating that 1-3 exert varying degrees of inhibition on tyrosinase-dependent melanin biosynthesis, and therefore, are candidates as skin-whitening agents.