YangoninCAS No.:500-62-9 |
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1460 |
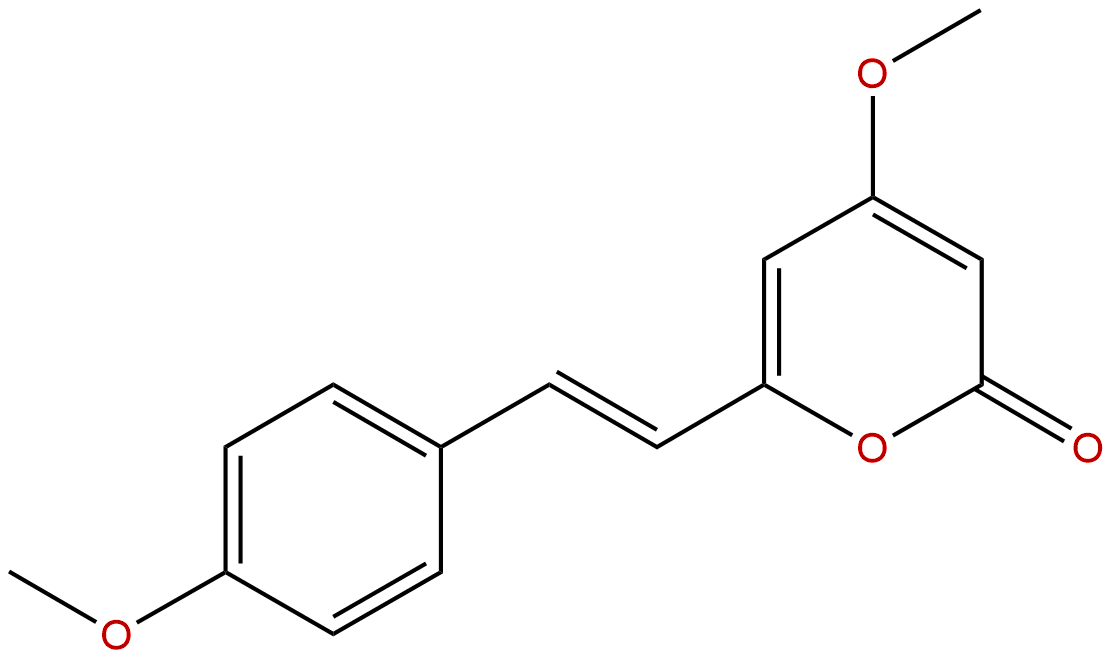
| Formula: | C15H14O4 |
| Mol Weight: | 258.273 |
| Botanical Source: | Piper methysticum Forst |
Product name: Yangonin
Synonym name:
Catalogue No.: BP1460
Cas No.: 500-62-9
Formula: C15H14O4
Mol Weight: 258.273
Botanical Source: Piper methysticum Forst
Physical Description:
Type of Compound: Phenols
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Yangonin is a novel CB₁ receptor ligand, it exhibits affinity for the human recombinant CB₁ receptor, it is also an effective inhibitor of EV-A71 infection in the low-micromolar. Yangonin could be a valuable candidate for the intervention of NF-κB-dependent pathological conditions such as inflammation.Yangonin induces autophagy and sensitizes bladder cancer cells to flavokawain A and docetaxel via inhibition of the mTOR pathway.
References:
J Pharmacol Sci. 2012;118(4):447-54.
Yangonin blocks tumor necrosis factor-α-induced nuclear factor-κB-dependent transcription by inhibiting the transactivation potential of the RelA/p65 subunit.
The nuclear factor-κB (NF-κB) transcription factors control many physiological processes including inflammation, immunity, and apoptosis. In our search for NF-κB inhibitors from natural resources, we identified Yangonin from Piper methysticum as an inhibitor of NF-κB activation.
METHODS AND RESULTS:
In the present study, we demonstrate that Yangonin potently inhibits NF-κB activation through suppression of the transcriptional activity of the RelA/p65 subunit of NF-κB. This compound significantly inhibited the induced expression of the NF-κB-reporter gene. However, this compound did not interfere with tumor necrosis factor-α (TNF-α)-induced inhibitor of κBα (IκBα) degradation, p65 nuclear translocation, and DNA-binding activity of NF-κB. Further analysis revealed that Yangonin inhibited not only the induced NF-κB activation by overexpression of RelA/p65, but also transactivation activity of RelA/p65. Moreover, Yangonin did not inhibit TNF-α-induced activation of p38, but it significantly impaired activation of extracellular signal-regulated kinase 1/2 and stress-activated protein kinase/c-Jun NH(2)-terminal kinase. We also demonstrated that pretreatment of cells with this compound prevented TNF-α-induced expression of NF-κB target genes, such as interleukin 6, interleukin 8, monocyte chemotactic protein 1, cyclooxygenase-2 and inducible nitric oxide.
CONCLUSIONS:
Taken together, Yangonin could be a valuable candidate for the intervention of NF-κB-dependent pathological conditions such as inflammation.
Antiviral Res. 2017 Jul;143:85-96.
Characterization of three small molecule inhibitors of enterovirus 71 identified from screening of a library of natural products.
Enterovirus 71 (EV-A71) is a major cause of hand, foot, and mouth disease (HFMD). Infection with EV-A71 is more often associated with neurological complications in children and is responsible for the majority of fatalities, but currently there is no approved antiviral therapy for treatment.
METHODS AND RESULTS:
Here, we identified auraptene, formononetin, and Yangonin as effective inhibitors of EV-A71 infection in the low-micromolar range from screening of a natural product library. Among them, formononetin and Yangonin selectively inhibited EV-A71 while auraptene could inhibit viruses within the enterovirus species A. Time of addition studies showed that all the three inhibitors inhibit both attachment and postattachment step of entry. We found mutations conferring the resistance to these inhibitors in the VP1 and VP4 capsid proteins and confirmed the target residues using a reverse genetic approach. Interestingly, auraptene- and formononetin-resistant viruses exhibit cross-resistance to other inhibitors while Yangonin-resistant virus still remains susceptible to auraptene and formononetin. Moreover, auraptene and formononetin, but not Yangonin protected EV-A71 against thermal inactivation, indicating a direct stabilizing effect of both compounds on virion capsid conformation. Finally, neither biochanin A (an analog of formononetin) nor DL-Kavain (an analog of Yangonin) exhibited anti-EV-A71 activity, suggesting the structural elements required for anti-EV-A71 activity.
CONCLUSIONS:
Taken together, these compounds could become potential lead compounds for anti-EV-A71 drug development and also serve as tool compounds for studying virus entry.
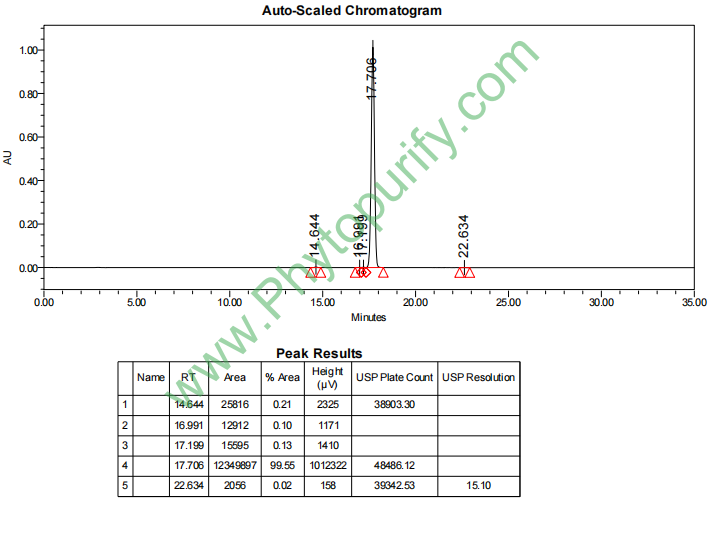
HPLC of Yangonin
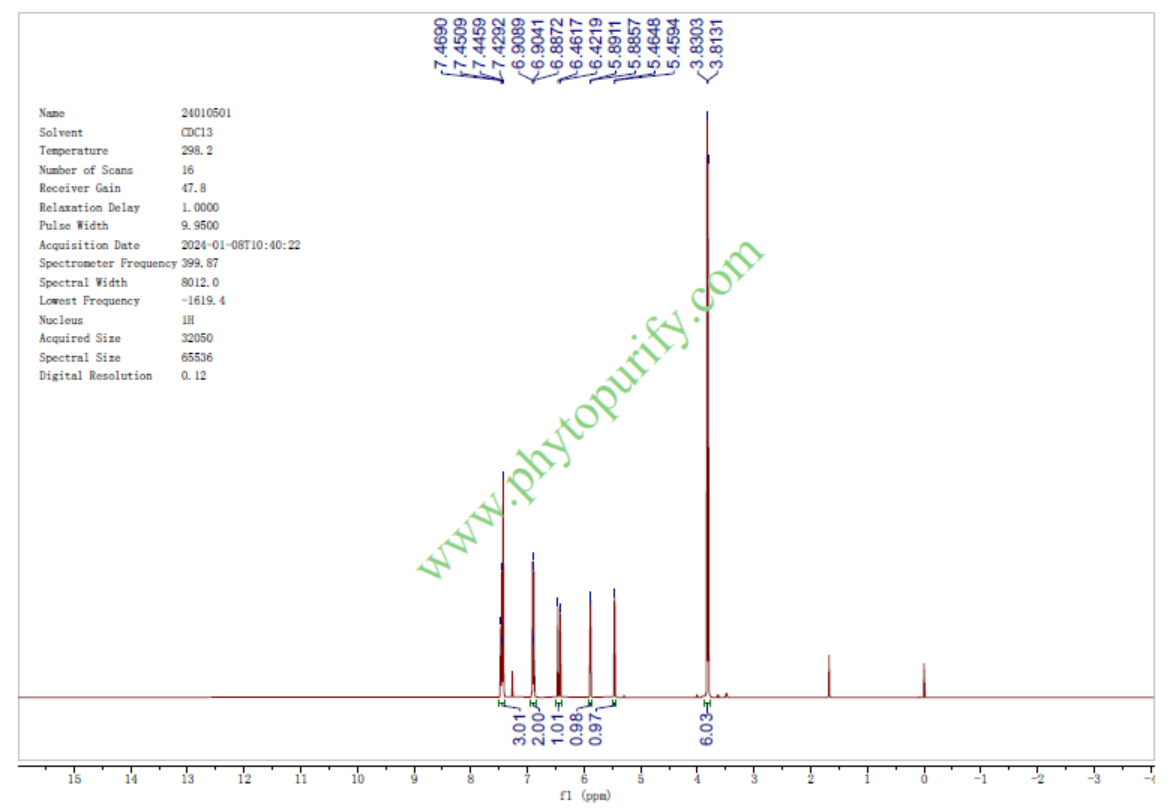
HNMR of Yangonin