
Isoastragaloside ICAS No.:84676-88-0 |
||||||||||
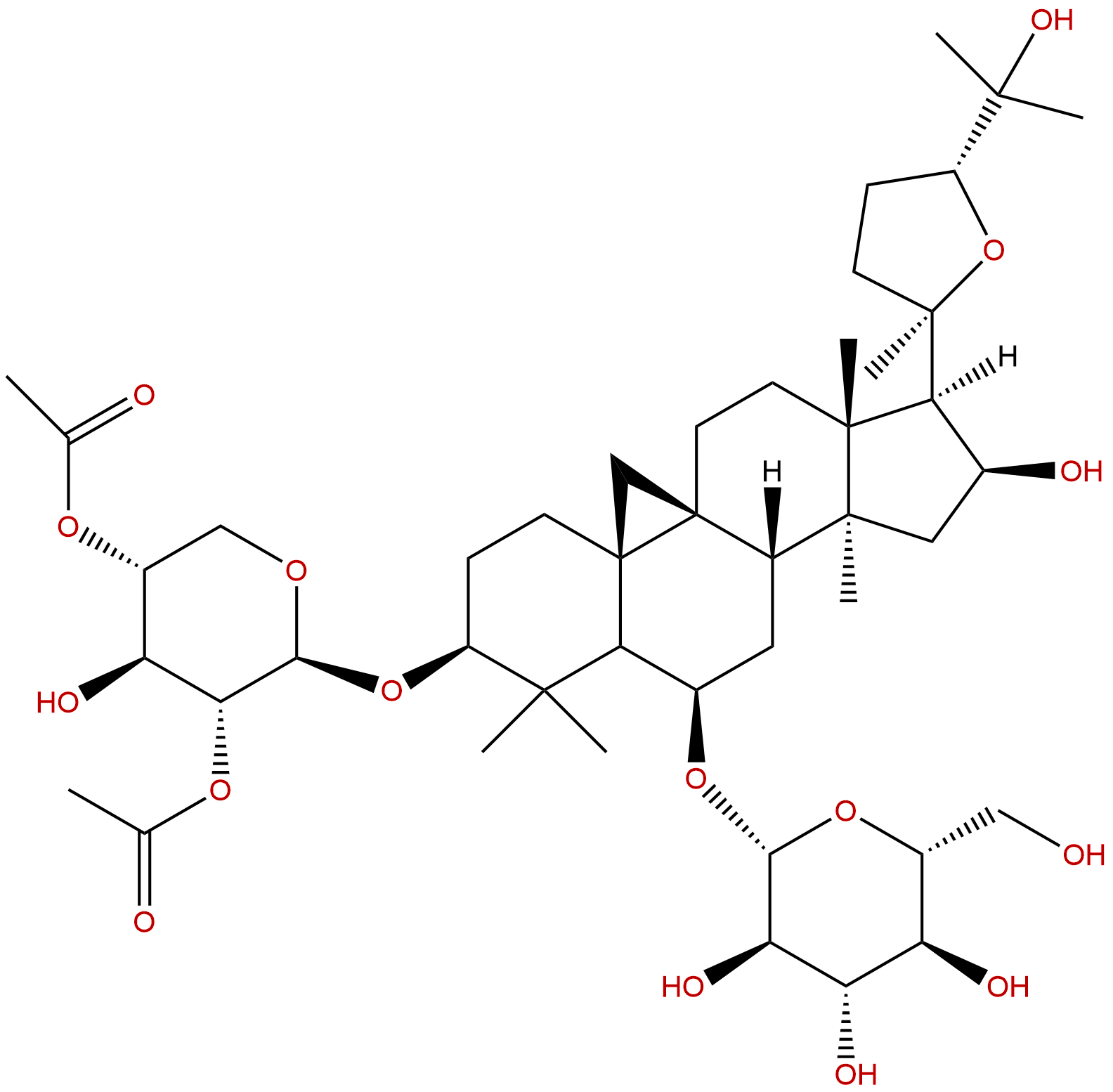 |
|
|
||||||||

| Catalogue No.: | BP1719 |
| Formula: | C45H72O16 |
| Mol Weight: | 869.055 |
| Botanical Source: | Astragali radix |
Product name: Isoastragaloside I
Synonym name:
Catalogue No.: BP1719
Cas No.: 84676-88-0
Formula: C45H72O16
Mol Weight: 869.055
Botanical Source: Astragalus membranaceus
Physical Description:
Type of Compound: Triterpenoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
The product could be supplied from milligrams to grams. Inquire for bulk scale.
We provide solution to improve the water-solubility of compounds, thereby facilitating the variety of activity tests and clinic uses.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
Isoastragaloside I may be promising in modulating CCl4-induced lethality and most of its toxic effects. Astragaloside II and Isoastragaloside I are sufficient to ameliorate insulin resistance, they may provide the lead as a novel class of therapeutics for obesity-related diseases.
References:
J. Med. Sci., 2002,2(3):119-23.
Phytochemical Investigation of Biologically Active Fractions of Astragalus spinosus Roots Grown in Egypt
The research work was undertaken to asses activity directed isolation of plant extracts, column fractions and isolated glycosides of Astragalus spinosus roots (Fabaceae) in the treatment of induced hepatic, renal and cardiac toxicities.
METHODS AND RESULTS:
A single subcutaneous dose of CCl4 (5.93 ml kg-1 b.wt. which represents 24h LD100) served as a toxicant, was used during this study. Ethyl acetate fraction was the most active fraction, then butanol, aqueous and dichloromethane fraction. By subjecting the ethyl acetate fraction to column chromatography (CC) it afforded two column fractions A and B which have effect in reducing CCl4-induced mortalities. Rechromatography of fractions A and B on medium pressure liquid chromatography (MPLC) and CC, respectively, afforded (a mixture of Isoastragaloside I and trigonoside I) and cycloastragenol-6-0-glucoside. The structure of the isolated glycosides was determined by 1H, 13C NMR, EIMS and FABMS spectral data, together with co-TLC with reference materials. The efficacy of the isolated glycosides (Isoastragaloside I and trigonoside I), cycloastragenol-6-0-glycoside and adsurgenic acid (recently isolated from the ether extract) in reducing CCl4-induced mortalities and some disturbances in biochemical parameters (liver transaminases AST, ALT and creatine kinase CK activities, serum urea and serum creatinine) were investigated in adult female Bufo regularis (Egyptian toads). The bioassay studies revealed that the effect of the glycosides on reducing CCl4-induced mortality have the following order (cycloastragenol-6-0-glycoside > Isoastragaloside I and trigonoside I > adsurgenic acid. The biochemical studies confirmed the bioassay studies.
CONCLUSIONS:
This is the first report on the identification of trigonoside I in Astragalus spinosus plant and the presence of Isoastragaloside I and cylcoastragenol-6-0-glycoside in A. spinosus roots. This suggested that these saponin glycosides may be promising in modulating CCl4-induced lethality and most of its toxic effects.