
TriptophenolideCAS No.:74285-86-2 |
||||||||||
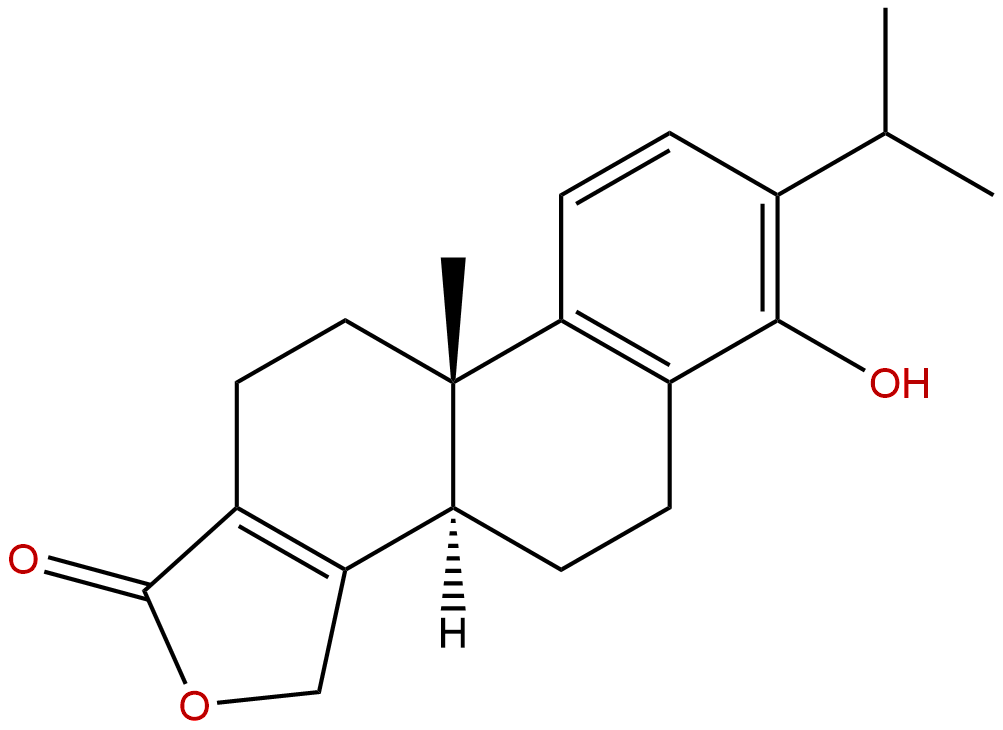 |
|
|
||||||||

| Catalogue No.: | BP1413 |
| Formula: | C20H24O3 |
| Mol Weight: | 312.409 |
| Botanical Source: | Tripterygium wilfordii Hook.f. |
Synonym name: Hypolide
Catalogue No.: BP1413
Cas No.: 74285-86-2
Formula: C20H24O3
Mol Weight: 312.409
Botanical Source: Tripterygium hypoglaucum and Tripterygium wilfordii
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Description:
Triptophenolide is a natural product from Tripterygium wilfordii Hook. f.
References:
Yao Xue Xue Bao. 1992;27(11):867-70.
The crystal structure of triptophenolide revisited.
Triptophenolide, C20H24O3, has been assigned two isomeric structures. DF Yu et al reported the X-ray analytical structure II in 1990. In 1982, however, FX Deng et al had already reported the spectrometric assignment of structure I. Now, we report again the single crystal analysis of Triptophenolide (I). The crystal belongs to space group P2(1)2(1)2(1) of orthorhombic system, with a = 7.270(6), b = 12.509(8), c = 36.20(1) A, Z = 8. The structure was solved by direct method and refined by the full-matrix least-square method to a final R factor 0.055, based on 2322 intensities with I > or = 3 sigma (I). It is highly probable that the result of DF Yu was in error with only 1292 available intensities.
J Org Chem. 2000 Apr 7;65(7):2208-17.
Enantioselective total synthesis of (-)-triptolide, (-)-triptonide, (+)-triptophenolide, and (+)-triptoquinonide.
The first enantioselective total synthesis of (-)-triptolide (1), (-)-triptonide (2), (+)-Triptophenolide (3), and (+)-triptoquinonide (4) was completed. The key step involves lanthanide triflate-catalyzed oxidative radical cyclization of (+)-8-phenylmenthyl ester 30 mediated by Mn(OAc)3, providing intermediate 31 with good chemical yield (77%) and excellent diastereoselectivity (dr 38:1). (+)-Triptophenolide methyl ether (5) was then prepared in > 99% enantiomeric excess (> 99% ee), and readily converted to natural products 1-4. In addition, transition state models were proposed to explain the opposite chiral induction observed in the oxidative radical cyclization reactions of chiral beta-keto esters 17 (without an alpha-substituent) and 17a (with an alpha-chloro substituent).