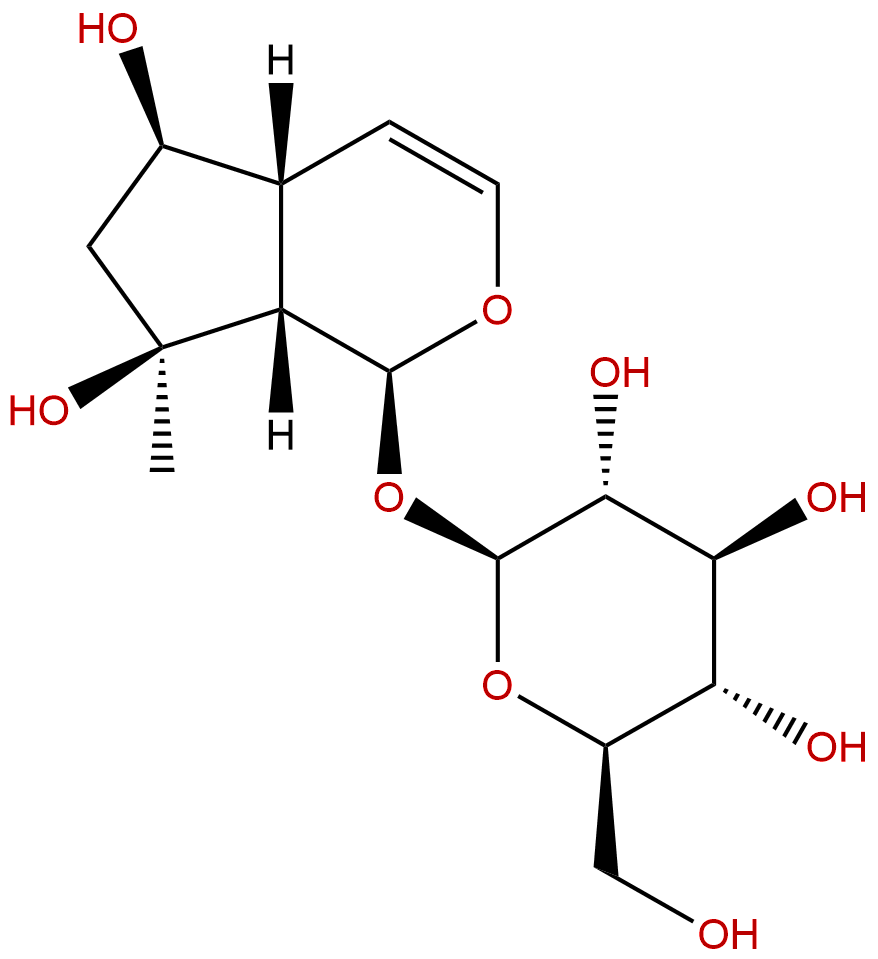
LeonurideCAS No.:52949-83-4
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0130 |
| Formula: | C15H24O9 |
| Mol Weight: | 348.348 |
Synonym name: Ajugol; Leonuride
Catalogue No.: BP0130
Cas No.: 52949-83-4
Formula: C15H24O9
Mol Weight: 348.348
Botanical Source: Ajuga reptans, Melittis melissophyllum, Rehmannia glutinosa and Leonurus cardiaca
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Description:
Ajugol shows some trypanocidal potential against Trypanosoma brucei rhodesiense (IC50 values 29.3–73.0 ug/ml).
References:
Phytochemistry, 2005, 66(3):355-62.Anti-protozoal and plasmodial FabI enzyme inhibiting metabolites of Scrophularia lepidota roots.
The ethanolic root extract of Scrophularia lepidota, an endemic plant of the Turkish flora, has been investigated for its anti-protozoal and inhibitory effect towards plasmodial enoyl-ACP reductase (FabI), a key enzyme of fatty acid biosynthesis in Plasmodium falciparum.
METHODS AND RESULTS:
Chromatographic separation of the extract yielded 10 iridoids (1-10), two of which are new, and a known phenylethanoid glycoside (11). The structures of the new compounds were determined as 3,4-dihydro-methylcatalpol (8) and 6-O-[4''-O-trans-(3,4-dimethoxycinnamoyl)-alpha-L-rhamnopyranosyl]aucubin (scrolepidoside, 9) by spectroscopic means. The remaining metabolites were characterized as catalpol (1), 6-O-methylcatalpol (2), aucubin (3), 6-O-alpha-L-rhamnopyranosyl-aucubin (sinuatol, 4), 6-O-beta-D-xylopyranosylaucubin (5), Ajugol (6), ajugoside (7), an iridoid-related aglycone (10) and angoroside C (11). Nine isolates were active against Leishmania donovani, with the new compound 9 being most potent (IC50 6.1 microg/ml). Except for 4, all pure compounds revealed some trypanocidal potential against Trypanosoma brucei rhodesiense (IC50 values 29.3-73.0 microg/ml). Only compound 10 showed moderate anti-plasmodial (IC50 40.6 microg/ml) and FabI enzyme inhibitory activity (IC50 100 microg/ml). 10 is the second natural product inhibiting the fatty acid biosynthesis of Plasmodium falciparum.
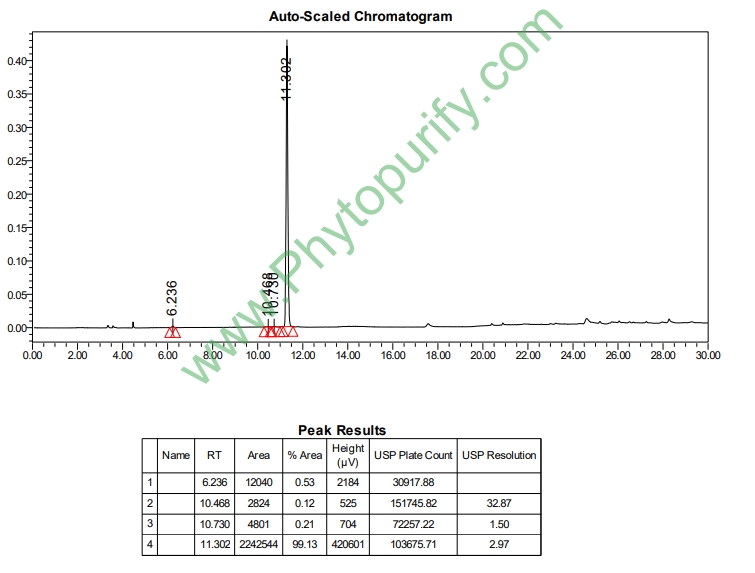
HPLC of Ajugol