
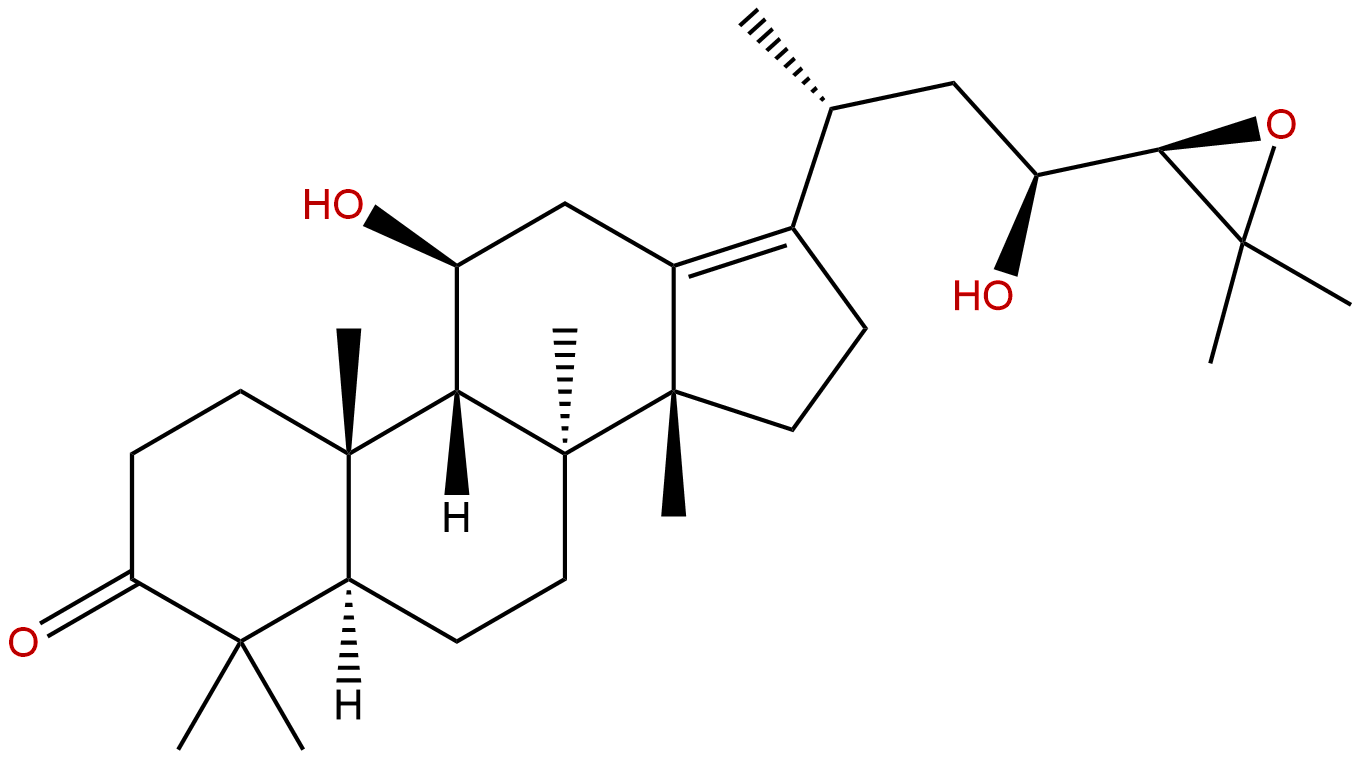
Alisol BCAS No.:18649-93-9
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP3059 |
| Formula: | C30H48O4 |
| Mol Weight: | 472.71 |
Product name: Alisol B
Synonym name:
Catalogue No.: BP3059
Cas No.: 18649-93-9
Formula: C30H48O4
Mol Weight: 472.71
Botanical Source:
Physical Description: Cryst.
Type of Compound: Triterpenoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
The product could be supplied from milligrams to grams. Inquire for bulk scale.
We provide solution to improve the water-solubility of compounds, thereby facilitating the variety of activity tests and clinic uses.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
Alisol B, a novel inhibitor of the sarcoplasmic/endoplasmic reticulum Ca(2+) ATPase pump, induces autophagy, endoplasmic reticulum stress, and apoptosis.Alisol B may be a potential novel therapeutic molecule for bone disorders through targeting the differentiation of osteoclasts as well as their functions. Alisol B also inhibited RANKL-induced expression of NFATc1 and c-Fos, which are key transcription factors for osteoclastogenesis.
References:
Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012 Oct;32(10):1407-12.
Alisol B inhibited complement 3a-induced human renal tubular epithelial to mesenchymal transition
To study whether Alisol B could inhibit complement 3a (C3a) induced renal tubular epithelial-mesenchymal transition (EMT).
METHODS AND RESULTS:
The in vitro cultured human renal tubular epithelial HK-2 cells were intervened with 5 ng/mL transforming growth factor-beta (TGF-beta), 0.1 micromol C3a, and 0.1 micromol C3a + 10 micromol Alisol B, respectively. The mRNA and protein expressions of alpha-SMA, E-cadherin, and C3 were detected using RT-PCR, Western blot, and immunofluorescence, respectively. The mRNA and protein expressions of C3 in HK-2 cells were up-regulated after intervention of C3a (P < 0.01), the mRNA and protein expressions of alpha-SMA in HK-2 cells were obviously enhanced (P < 0.01), the mRNA and protein expressions of E-cadherin obviously decreased (P < 0.01). When compared with the group intervened by exogenous C3a, after intervention of Alisol B, the mRNA and protein expressions of alpha-SMA in HK-2 cells were obviously reduced (P < 0.01), the mRNA and protein expressions of E-cadherin obviously increased (P < 0.05).
CONCLUSIONS:
Exogenous C3a could induce renal tubular EMT. Alisol B was capable of suppressing C3a induced EMT.
Biochem Pharmacol. 2010 Aug 1;80(3):352-61.
Alisol-B, a novel phyto-steroid, suppresses the RANKL-induced osteoclast formation and prevents bone loss in mice.
Osteoclasts, bone-resorbing multinucleated cells, are differentiated from hemopoietic progenitors of the monocyte/macrophage lineage. Bone resorption by osteoclasts is considered a potential therapeutic target to the treatment of erosive bone diseases, including osteoporosis, rheumatoid arthritis, and periodontitis.
METHODS AND RESULTS:
In the present study, we found that Alisol B, a phyto-steroid from Alisma orientale Juzepczuk, exhibited inhibitory effects on osteoclastogenesis both in vitro and in vivo. Although RT-PCR analysis showed that Alisol B did not affect the 1alpha,25(OH)(2)D(3)-induced expressions of RANKL, OPG and M-CSF mRNAs in osteoblasts, addition of Alisol B to co-cultures of mouse bone marrow cells and primary osteoblasts with 10(-8)M 1alpha,25(OH)(2)D(3) caused significant inhibition of osteoclastogenesis. We further examined the direct effects of Alisol B on osteoclast precursors. Alisol B strongly inhibited RANKL-induced osteoclast formation when added during the early stage of cultures, suggesting that Alisol B acts on osteoclast precursors to inhibit RANKL/RANK signaling. Among the RANK signaling pathways, Alisol B inhibited the phosphorylation of JNK, which are upregulated in response to RANKL in bone marrow macrophages, Alisol B also inhibited RANKL-induced expression of NFATc1 and c-Fos, which are key transcription factors for osteoclastogenesis. In addition, Alisol B suppressed the pit-forming activity and disrupted the actin ring formation of mature osteoclasts. In a hypercalcemic mouse model induced by 2-methylene-19-nor-(20S)-1alpha,25(OH)(2)D(3) (2MD), an analog of 1alpha,25(OH)(2)D(3), administration of Alisol Bsignificantly suppressed 2MD-induced hypercalcemia as resulting from the inhibition of osteoclastogenesis.
CONCLUSIONS:
Taken together, these findings suggest that Alisol B may be a potential novel therapeutic molecule for bone disorders by targeting the differentiation of osteoclasts as well as their functions.
HPLC of Alisol B
