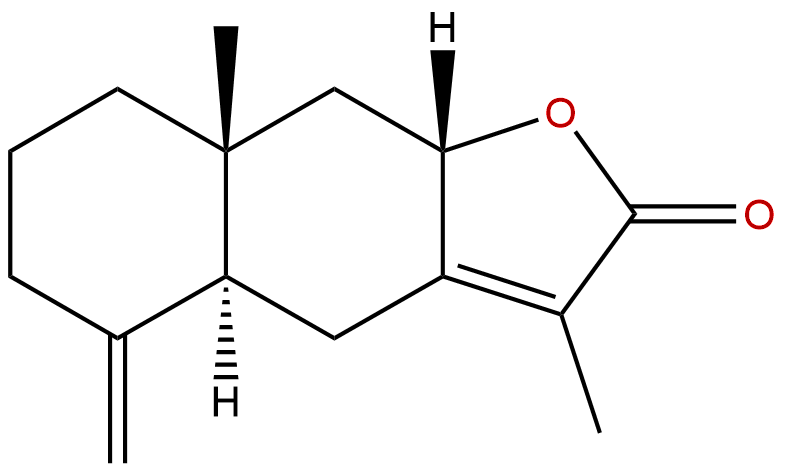
Atractylenolide IICAS No.:73069-14-4
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0215 |
| Formula: | C15H20O2 |
| Mol Weight: | 232.323 |
Product name: Atractylenolide II
Synonym name: Asterolide
Catalogue No.: BP0215
Cas No.: 73069-14-4
Formula: C15H20O2
Mol Weight: 232.323
Botanical Source: Atractylodes macrocephala
Physical Description:
Type of Compound: Sesquiterpenoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Atractylenolide II has antiinflammatory activity, it can inhibit platelets activities and thrombus formation. Atractylenolide II has cytotoxic/apoptotic effects may via p38 activation ,ERK and Akt inactivation, p53 dependent, it also has antimelanoma effect by inhibiting STAT3 signalling.
References:
J. Am. Coll. Cardiol., 2015, 66(16):C44-C44.
GW26-e1245 Atractylenolide II and Atractylenolide III Inhibit Platelets Activities and Thrombus Formation
Atractylenolide II and Atractylenolide III Inhibit Platelets Activities and Thrombus Formation.
Biomed Chromatogr. 2007 Mar;21(3):299-303.
Determination of atractylenolide II in rat plasma by reversed-phase high-performance liquid chromatography.
METHODS AND RESULTS:
A method for quantitative determination of Atractylenolide II in rat plasma using reversed-phase high-performance liquid chromatography (RP-HPLC) coupled with UV spectrometry was established. From a variety of compounds and solvents tested, Atractylenolide III was selected as the internal standard (IS) and ethyl acetate was found to be the best solvent for extracting Atractylenolide II from plasma samples. RP-HPLC analysis of the extracts was performed on an analytical column (DIKMA ODS, 150 x 4.6 mm; i.d., 5 microm) equipped with a security guard pre-column system. There was good linearity over the range 0.05-5.0 microg/mL (r > 0.99). The recoveries were more than 90.0% in plasma, and the intra- and inter-day coefficients of variation were less than 10.0% in all cases. The limit of detection (LOD) was 0.025 microg/mL and the lower limit of quantification (LLOQ) was 0.05 microg/mL.
CONCLUSIONS:
The RP-HPLC method was applied to quantitate Atractylenolide II in rat plasma within 24 h in a pharmacokinetics study where experimental rats received a single dose of Atractylenolide II (60 mg/kg).
Phytotherapy Research, 2007, 21(4):347-353.
Antiinflammatory Principles of Atractylodes Rhizomes.
The crude drug"jutsu"prepared from Atractylodes rhizomes has been used for antiinflammatory purposes in Oriental medicine.
METHODS AND RESULTS:
In fact, a preparation from A. japonica was found to show significant inhibition of the increased vascular permeability induced by acetic acid. Fractionation of the extract, monitoring by bioassay, resulted in the isolation of two active principles, (+)-eudesma-4 (14), 7 (11)-dien-8-one (VI) and atractylenolide I (VII).
CONCLUSIONS:
The structurally related principles Atractylenolide II and III (VIII and IX) also had the tendency to show antiinflammatory activity.
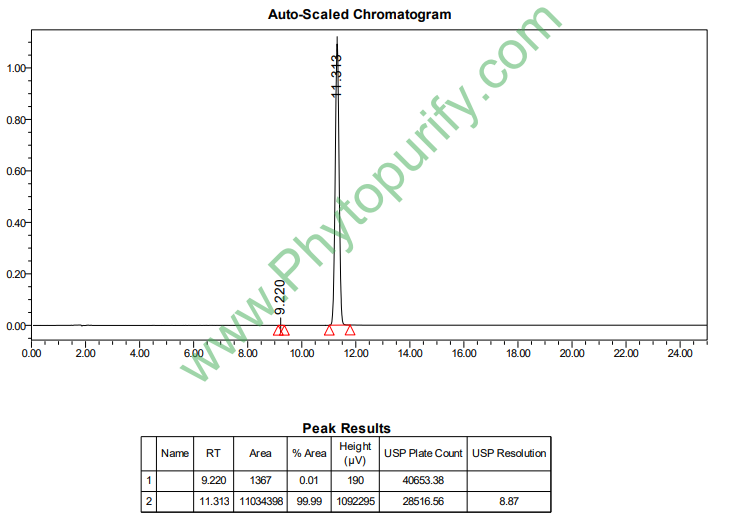
HPLC of Atractylenolide II