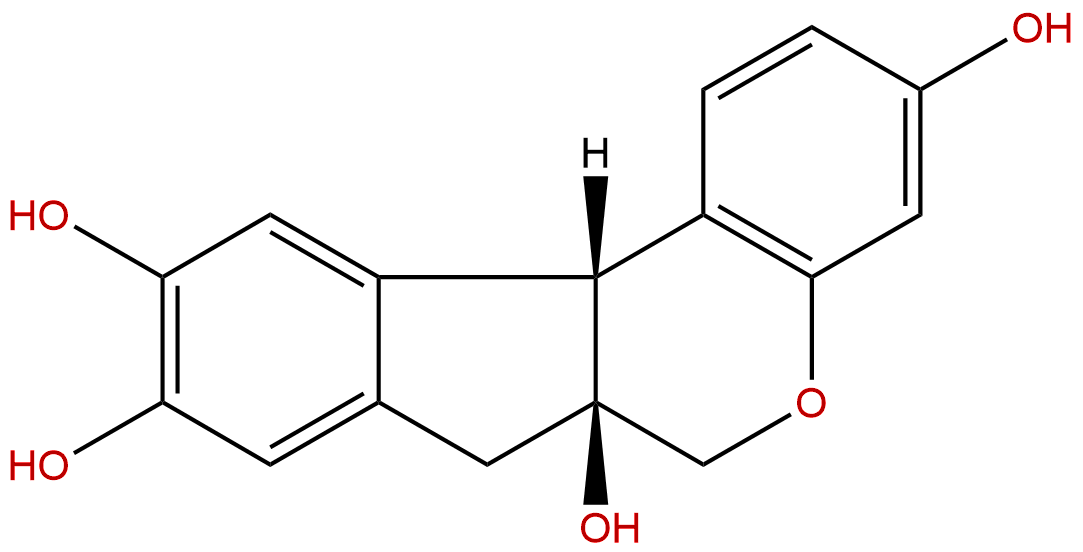
Brazilin Descrtption
Product name: Brazilin
Synonym name:
Catalogue No.: BP0281
Cas No.: 474-07-7
Formula: C16H14O5
Mol Weight: 286.283
Botanical Source:
Physical Description: Red powder
Type of Compound: Flavonoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams, up to kilograms
Inquire for bulk scale.
Descriptions:
Brazilin, isolated from Caesalpinia sappan has been known as a natural red pigment, exhibits the inhibitory effect on lipopolysaccharide (LPS)-stimulated NO production in a dose-dependent manner (IC50=24.3 microM), might be associated with the regulation of transcription factors NF-kappaB and AP-1; inducible isoform of nitric oxide synthase (NOS) plays an important role in inflammation and carcinogenesis, suggests that the suppressive effect of iNOS gene expression by brazilin might provide one possible mechanism for its anti-inflammatory and cancer chemopreventive activity. [1]
Brazilin exhibits anti-hepatotoxicity, antiplatelet activity, and anti-inflammatory activities, it also inhibits UVB-induced MMP-1/3 expressions and secretions by suppressing of NF-κB activation in human dermal fibroblasts, thus, it might be used as a potential agent for treatment of UV-induced skin photoaging.[2]
Brazilin protects the cells against t -butyl hydroperoxide ( t -BHP)-induced cell death, and the protective effect was abrogated by anti-sense oligodeoxynucleotides (ODN) against the HO-1 gene, suggests that the expression of HO-1 by brazilin is mediated via the PI3K/Akt and ERK pathways, and this expression inhibits t -BHP-induced cell death in House Ear Institute-Organ of Corti 1 (HEI-OC1) cells.[3]
Brazilin induces vasorelaxation by the increasing intracellular Ca(2+) concentration in endothelial cells of blood vessels and hence activating Ca(2+)/calmodulin-dependent NO synthesis, the NO is released and then transferred into smooth muscle cells to activate guanylyl cyclase and increase cGMP content, resulting in vasorelaxation.[4]
Brazilin, the main principle of Caesalpinia sappan, was able to improve the altered immune functions caused by halothane administration in mice.[5]
Brazilin induces apoptosis and G2/M arrest via inactivation of histone deacetylase in multiple myeloma U266 cells, suggests that it has potential as a chemotherapeutic agent alone or in combination with an anticancer agent for multiple myeloma treatment.[6]
Brazilin shows dose-dependent inhibition of cell proliferation and induction of apoptosis in glioma cells, it also increases the ratio of cleaved poly-(ADP)-ribose polymerase and decreases the expression of caspase-3 and caspase-7.[7]
Brazilin has anti-IKK activity , can selectively disrupt proximal IL-1 receptor signaling complex formation by targeting an IKK-upstream signaling components.[8]
References:
[1] Bae I K, Min H Y, Han A R, et al. Eur J Pharmacol, 2005, 513(3):237-42.
[2] Lee Y R, Noh E M, Han J H, et al. Eur J Pharmacol, 2012, 674(2–3):80-6.
[3] Choi B M, Kim B R. Eur J Pharmacol, 2008, 580(1–2):12-8.
[4] Hu C M, Kang J J, Chen C L, et al. Eur J Pharmacol, 2003, 468(1):37-45.
[5] Choi S Y, Yang K M, Jeon S D, et al. Planta Medica, 1997, 63(5):405-8.
[6] Kim B, Kim S H, Jeong S J, et al. J Agr Food Chem, 2012, 60(39):9882-9.
[7] Lee D Y, Lee M K, Kim G S, et al. Molecules, 2013, 18(2):2449-57.
[8] Jeon J, Ji H L, Park K A, et al. Biochem Pharmacol, 2014, 89(4):515-25.
[9] Zhao H X, Bai H, Wang Y S, et al. West China J Pharm Sci, 2010, 25(3):363-4.