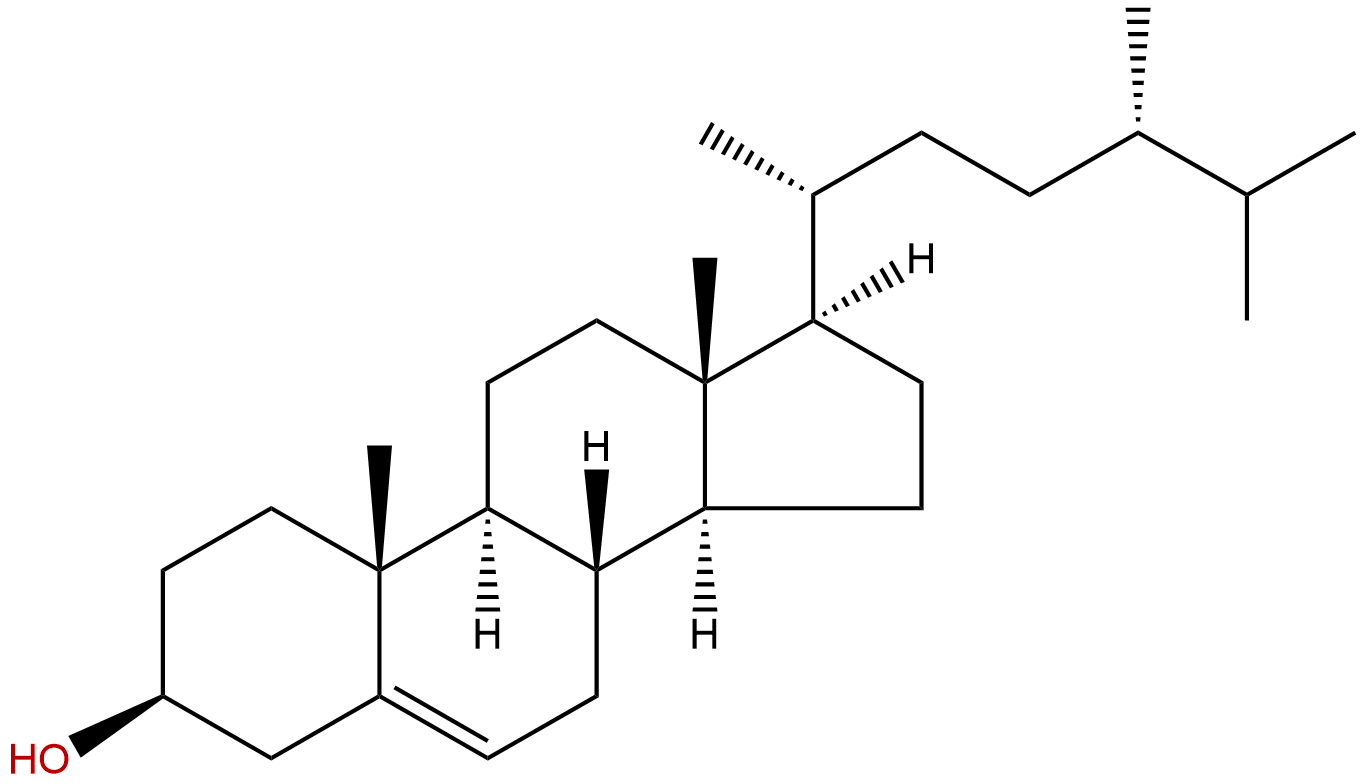
CampesterolCAS No.:474-62-4
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0307 |
| Formula: | C28H48O |
| Mol Weight: | 400.691 |
Product name: Campesterol
Synonym name: Mieyajunsu A; Campest-5-en-3-ol
Catalogue No.: BP0307
Cas No.: 474-62-4
Formula: C28H48O
Mol Weight: 400.691
Botanical Source:
Physical Description:
Type of Compound: Steroids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams, up to kilograms
Inquire for bulk scale.
Descriptions:
Dietary campest-5-en-3-one (campestenone), an oxidized derivative of campesterol, can activate PPAR alpha, promote energy consumption and significantly reduce visceral fat weight and the concentration of triacylglycerol in serum and liver of rats.[1]
Campesterol oxidised derivatives and dihydrobrassicasterol have toxicity in U937 and HepG2 cells.[2]
Campesterol oxidation products have different antioxidant effects.[3]
At higher sterol concentrations, campesterol (Camp) and brassicasterol (Bras) are less miscible and less effective than cholesterol (Chol) at ordering the hydrocarbon chains of the sterol-enriched fluid DPPC bilayers, overall, these alkyl side chain modifications generally reduce the ability of Chol to produce its characteristic effects on DPPC bilayer physical properties. [4]
References:
[1] Ikeda I, Konno R, Shimizu T, et al.BBA-BiomembranesI?, 2006, 1760(5):800-7.
[2] O Callaghan Y, Kenny O, O’Connell N M, et al. Biochimie, 2013, 95(3):496-503.
[3] Kmiecik D, Korczak J, Rudzińska M, et al. Food Chem, 2011, 128(4):937-42.
[4] Benesch M G K, Mcelhaney R N. BBA-BiomembranesI, 2014, 1838(7):1941-9.
[5] Jia M Q, Tang H, Cui-Hua L I, et al. Food Sci Technol 2013, 12(4):651.
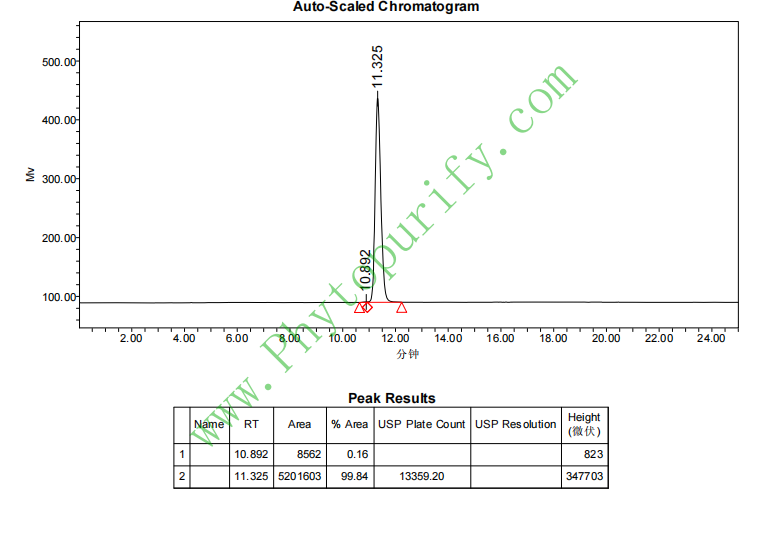
HPLC of Campesterol