
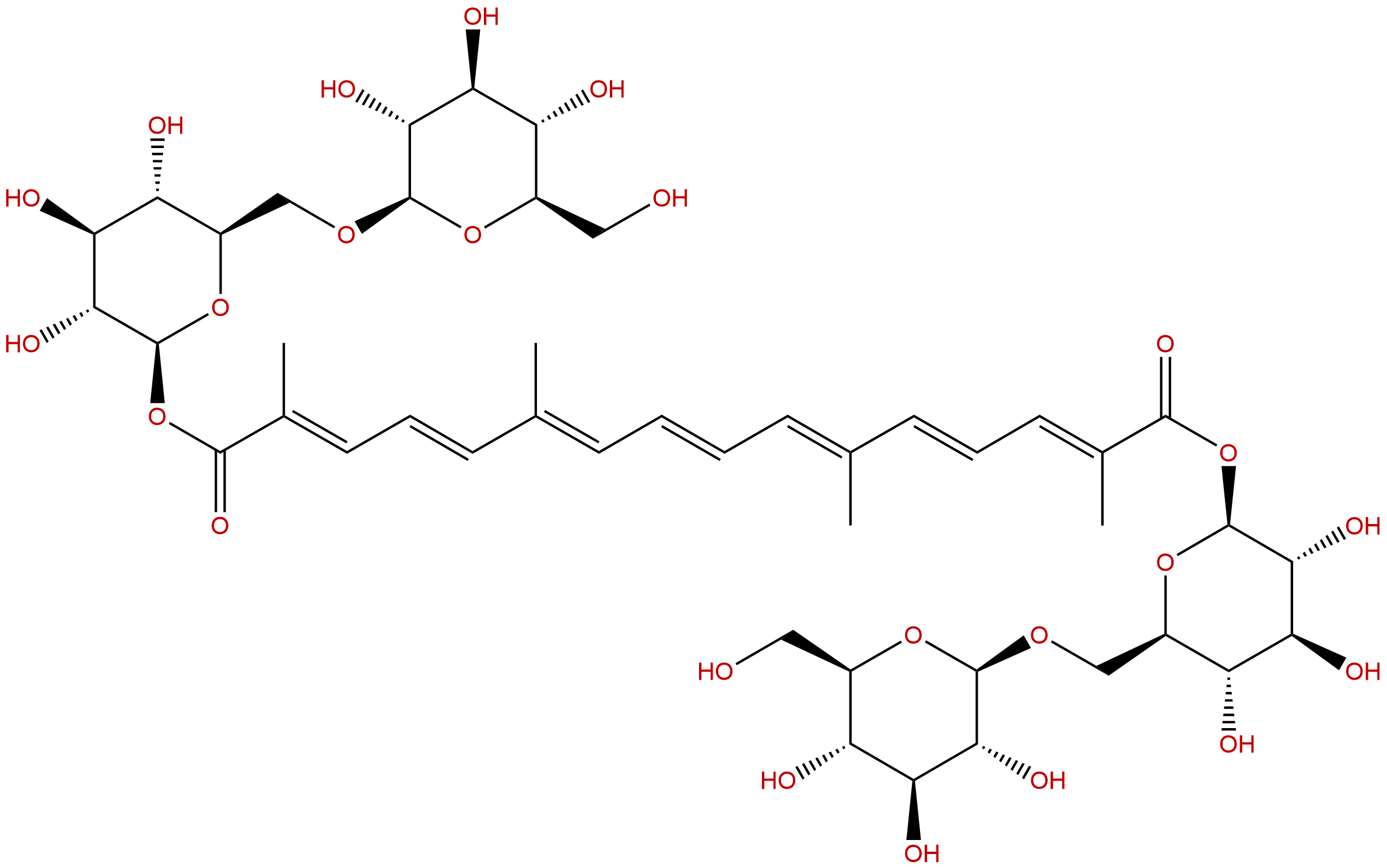
Crocin ICAS No.:42553-65-1
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0406 |
| Formula: | C44H64O24 |
| Mol Weight: | 976.972 |
Product name: Crocin I
Synonym name: alpha-Crocin; Crocin; Gardenin
Catalogue No.: BP0406
Cas No.: 42553-65-1
Formula: C44H64O24
Mol Weight: 976.972
Botanical Source: Crocus sativus L.; Fructus Gardeniae
Physical Description:
Type of Compound: Diterpenoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Crocin is a water-soluble carotenoid pigment of saffron (Crocus sativus L.), it has been used as a spice for flavoring and coloring food preparations. Crocin has anti-inflammatory, anti-oxidative, anti-apoptotic, anti-asthma, anti-cancer, and hypolipidemic effects. Crocin improves toxic effects of diazinon via reducing lipid peroxidation and restoring altered contractile and relaxant responses in rat aorta; it also protects retinal photoreceptors against light-induced cell death. Crocin can also promote ovarian cancer HO-8910 cell apoptosis, most likely by increasing p53 and Fas/APO-1 expression, and then activating the apoptotic pathway regulated by Caspase-3.
References:
Nat Prod Commun. 2015 Feb;10(2):249-52.
Ovarian cancer HO-8910 cell apoptosis induced by crocin in vitro.
The effect and mechanism of ovarian cancer HO-8910 cell apoptosis induced by Crocin.
METHODS AND RESULTS:
MTT assay was performed to detect the inhibitory action of Crocin on the proliferation of HO-8910 cells. Flow cytometry was used to test the cell cycle distribution and apoptosis rate of ovarian cancer HO-8910 cells. Western blot analysis was utilized to measure the levels of apoptotic proteins such as p53, Fas/APO-1, and Caspase-3. MTT analysis revealed that Crocin significantly inhibited the growth of HO-8910 cells. Additionally, flow cytometry illustrated that Crocin raised the proportion of HO-8910 cells in the G0/G1 phase and increased their apoptosis rate. Furthermore, Western blot analysis revealed that Crocin up-regulated the expression of p53, Fas/APO-1, and Caspase-3.
CONCLUSIONS:
The results of this study showed that Crocin can significantly inhibit the growth of HO-8910 cells and arrest them in the G0/G1 phase. Crocin can also promote ovarian cancer HO-8910 cell apoptosis, most likely by increasing p53 and Fas/APO-1 expression, and then activating the apoptotic pathway regulated by Caspase-3.
Invest Ophthalmol Vis Sci. 2006 Jul;47(7):3156-63.
Protective effect of crocin against blue light- and white light-mediated photoreceptor cell death in bovine and primate retinal primary cell culture.
The present study was performed to investigate the effect of Crocin on blue light- and white light-induced rod and cone death in primary retinal cell cultures.
METHODS AND RESULTS:
Primary retinal cell cultures were prepared from primate and bovine retinas. Fifteen-day-old cultures were exposed to blue actinic light or to white fluorescent light for 24 hours. Cultures were treated by the addition of different concentrations of Crocin for 24 hours before light exposure or for 8 hours after light exposure. Cultures kept in the dark were used as controls. Green nucleic acid stain assay was used to evaluate cell death. Rods and cones were immunolabeled with specific antibodies and counted. TUNEL labeling was used to detect fragmented DNA in fixed cells after light exposure. Primary retinal cell cultures contained a mixture of retinal cells enriched in photoreceptors, bipolar cells, and Müller cells. Twenty-four-hour exposure to blue and white light induced death in 70% to 80% of the photoreceptors in bovine and primate retinal cell cultures. Crocin protected the photoreceptors against blue light- or white light-mediated damage in a concentration-dependent manner with an EC50 of approximately 30 microM. TUNEL assays confirmed that Crocin protected photoreceptors from light damage.
CONCLUSIONS:
These results show that blue and white light selectively induce rod and cone cell death in an in vitro model. Crocin protects retinal photoreceptors against light-induced cell death.
Immunopharmacol Immunotoxicol. 2015 Jun;37(3):236-43.
Anti-asthma potential of crocin and its effect on MAPK signaling pathway in a murine model of allergic airway disease.
Crocin, a diterpenoid glucoside, has multitudinous activities such as anti-inflammation, anti-allergy, anti-oxidation and relaxing smooth muscles. In this study, the potential of Crocin as an anti-asthma agent was investigated in a murine model.
METHODS AND RESULTS:
BALB/c mice were sensitized and challenged by ovalbumin (OVA) to induce allergic airway inflammation, with Crocin administered one hour before every OVA challenge. Airway hyper-reactivity was evaluated by lung function analysis systems. Leukocyte counts in bronchoalveolar lavage fluid (BALF) were measured by a hemocytometer and Diff-Quick-stained smears. Lung tissues were stained with hematoxylin-eosin, Congo red and methylene blue for histopathological inspection. Inflammatory mediators in serum, BALF and lung were measured by ELISA or RT-PCR. Effects of Crocin on MAPK signaling pathways were investigated by western blot analysis. Crocin significantly suppressed airway inflammation and hyper-reactivity, reduced levels of BALF interleukin (IL-4), IL-5, IL-13 and tryptase, lung eosinophil peroxidase and serum OVA-specific IgE, and inhibited the expression of lung eotaxin, p-ERK, p-JNK and p-p38 in the OVA-challenged mice.
CONCLUSIONS:
These results demonstrated that the suppression of Crocin on airway inflammation and hyper-reactivity in a murine model, thus Crocin might have a great potential to be a candidate for the treatment of asthma.
Drug Chem Toxicol. 2014 Oct;37(4):378-83.
Protective effect of crocin on diazinon induced vascular toxicity in subchronic exposure in rat aorta ex-vivo.
Diazinon (DZN) is a widely used organophosphate insecticide. Although mechanism of DZN cardiovascular toxicity is primarily mediated through inhibition of acetylcholinesterase, however, DZN causes remarkable atropine-insensitive hypotension in rats. It has been proved that oxidative stress is an important mechanism of DZN toxicity especially in chronic exposure. Crocin, an active ingredient of saffron, has been found to antagonize the hypotensive effects of DZN in rats, but do not reverse acetylcholinesterase inhibition. In this study the effects of DZN on contractile and relaxant responses in rat aorta as well as ex-vivo antioxidant actions of Crocin have been investigated.
METHODS AND RESULTS:
Rats were divided into 7 groups: corn oil (control), DZN (15 mg/kg/day, gavage), Crocin (12.5, 25 and 50 mg/kg/day, i.p.) plus DZN, vitamin E (200 IU/kg, i.p., three days a week) plus DZN and Crocin (50 mg/kg/day, i.p.) groups. Treatments were continued for 4 weeks. Contractile and relaxant responses were evaluated on the isolated aorta. Our results showed that DZN not only decreased the contractile responses to KCl and Phenylephrine (PE) (p < 0.001), but also attenuated the relaxant response to acetylcholine (ACh) (p < 0.01). Crocin and vitamin E attenuated lipid peroxidation, improved the reduction of contractile responses by KCl and PE and restored the decrease in ACh relaxation in rat aorta.
CONCLUSIONS:
DZN induced vascular toxicity which may be due to oxidative stress and not to a cholinergic mechanism. Crocin improved toxic effects of DZN via reducing lipid peroxidation and restoring altered contractile and relaxant responses in rat aorta.
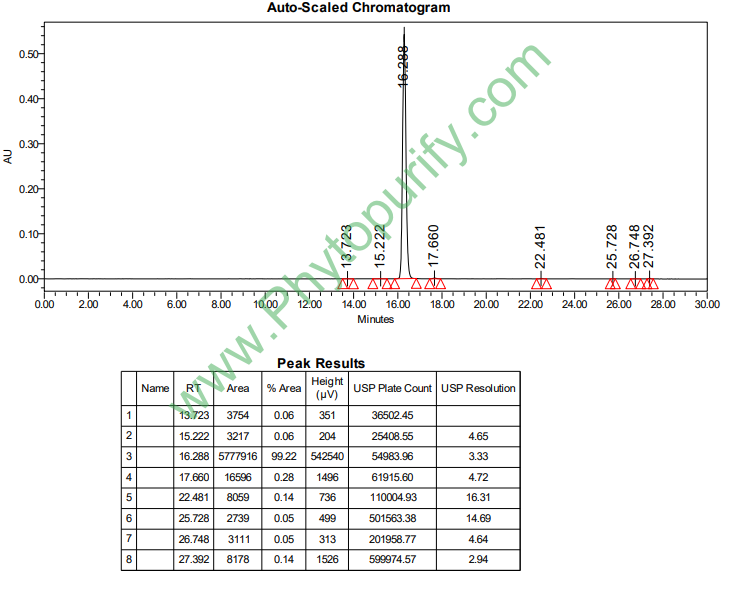
HPLC of Crocin I
HNMR of Crocin I
