
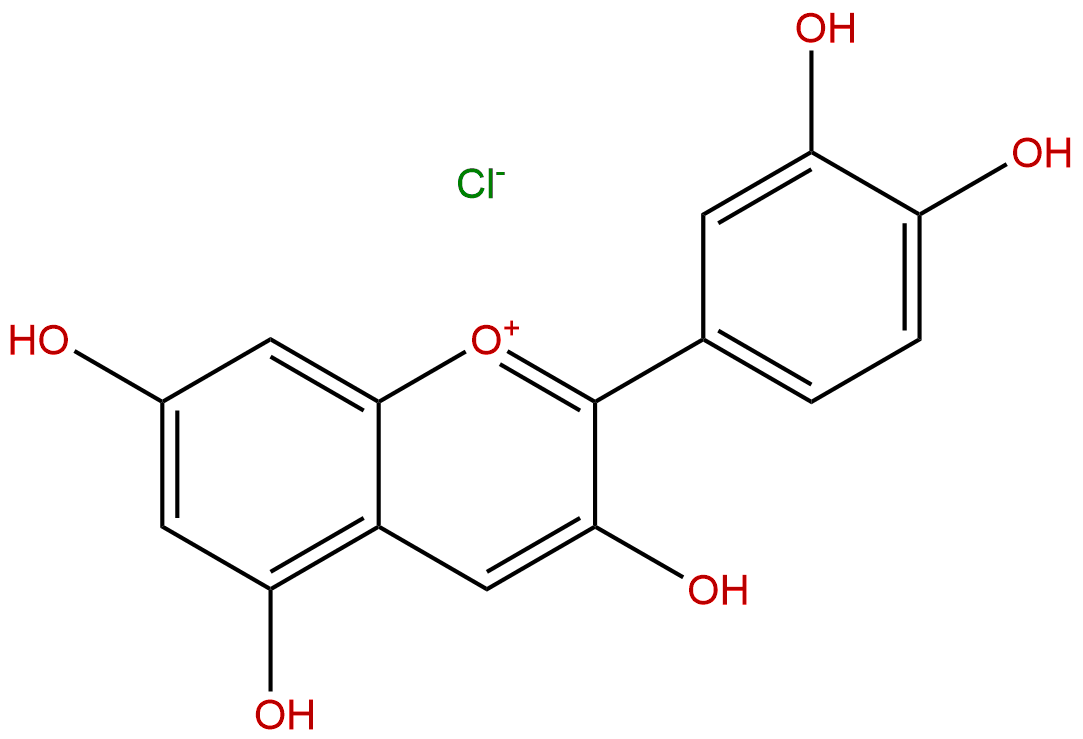
Cyanidin chlorideCAS No.:528-58-5
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0429 |
| Formula: | C15H11ClO6 |
| Mol Weight: | 322.697 |
Product name: Cyanidin chloride
Synonym name: Cyanidine; Cyanidol chloride
Catalogue No.: BP0429
Cas No.: 528-58-5
Formula: C15H11ClO6
Mol Weight: 322.697
Botanical Source: Black Rice
Physical Description:
Type of Compound: Anthocyanins
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Cyanidin Chloride, the main phenolic antioxidant in the grape (Vitis vinifera), in particular in the liposomal forms, could be used for treatment of diabetes mellitus complications. It has a dual effect on RANKL-induced osteoclastogenesis, exhibits therapeutic potential in prevention of osteoclasts related bone disorders.
References:
Biochem Pharmacol. 1996 Oct 11;52(7):1033-9.
Inhibition of lipid peroxidation and the active oxygen radical scavenging effect of anthocyanin pigments isolated from Phaseolus vulgaris L.
METHODS AND RESULTS:
No attention has been paid to anthocyanin pigments from the viewpoint of inhibitors of lipid peroxidation and scavengers of active oxygen radicals; therefore, we investigated the antioxidative, radical scavenging, and inhibitory effects on lipid peroxidation by UV light irradiation of three anthocyanin pigments, pelargonidin 3-O-beta-D-glucoside (P3G), cyanidin 3-O-beta-D-glucoside (C3G), and delphinidin 3-O-beta-D-glucoside (D3G), isolated from the Phaseolus vulgaris L. seed coat, and their aglycons, pelargonidin chloride (Pel), Cyanidin Chloride (Cy), and delphinidin chloride (Del).
CONCLUSIONS:
All pigments had strong antioxidative activity in a liposomal system and reduced the formation of malondialdehyde by UVB irradiation. On the other hand, the extent of antioxidative activity in a rat liver microsomal system and the scavenging effect of hydroxyl radicals (-OH) and superoxide anion radicals (O2-) were influenced by their own structures.
Planta Med. 2013 Nov;79(17):1599-604.
Treatment of diabetes in the mouse model by delphinidin and cyanidin hydrochloride in free and liposomal forms.
Cyanidin Chloride and delphinidin are the main phenolic antioxidants in the grape (Vitis vinifera). The aim of this study was to investigate the in vitro and in vivo inhibitory effects of delphinidin and Cyanidin Chloride in the free and liposomal forms on the albumin glycation reaction.
METHODS AND RESULTS:
Delphinidin and Cyanidin Chlorides were encapsulated in the liposomes using an extrusion method. The rate of albumin glycation was evaluated using the ELISA method. Finally, in vivo anti-glycation of delphinidin and Cyanidin Chloride in the free and liposomal forms in diabetic mice was investigated. The encapsulation efficacies of delphinidin and Cyanidin Chloride in the liposomes were 89.05 % ± 0.18 and 85.00 % ± 0.15, respectively. In vitro treatment with 100 mg/mL delphinidin and Cyanidin Chloride in free forms could reduce the rate of albumin glycation to 30.50 ± 3.46 and 46.00 ± 2.50 %, respectively. Under identical conditions, the delphinidin and Cyanidin Chloride-loaded liposomes could reduce the rate of albumin glycation to 8.50 ± 2.10 and 14.60 ± 3.60 %, respectively. In vivo testing showed that anti-glycation activity of delphinidin and cyanidin in loaded forms was higher than in free forms. The daily administration of 100 mg/kg delphinidin chloride-loaded liposomes to diabetic mice at eight weeks could decrease the rate of albumin and HbA1c glycation to 46.35 ± 1.20 and 3.60 ± 0.25 %, respectively. Moreover, under identical conditions, the loaded liposomes with Cyanidin Chloride could decrease the rate of albumin and HbA1c glycation to 55.56 ± 1.32 and 4.95 ± 0.20 %, respectively.
CONCLUSIONS:
The findings showed that delphinidin and Cyanidin Chloride, in particular in the liposomal forms, could be used for treatment of diabetes mellitus complications.
HPLC of Cyanidin chloride
