
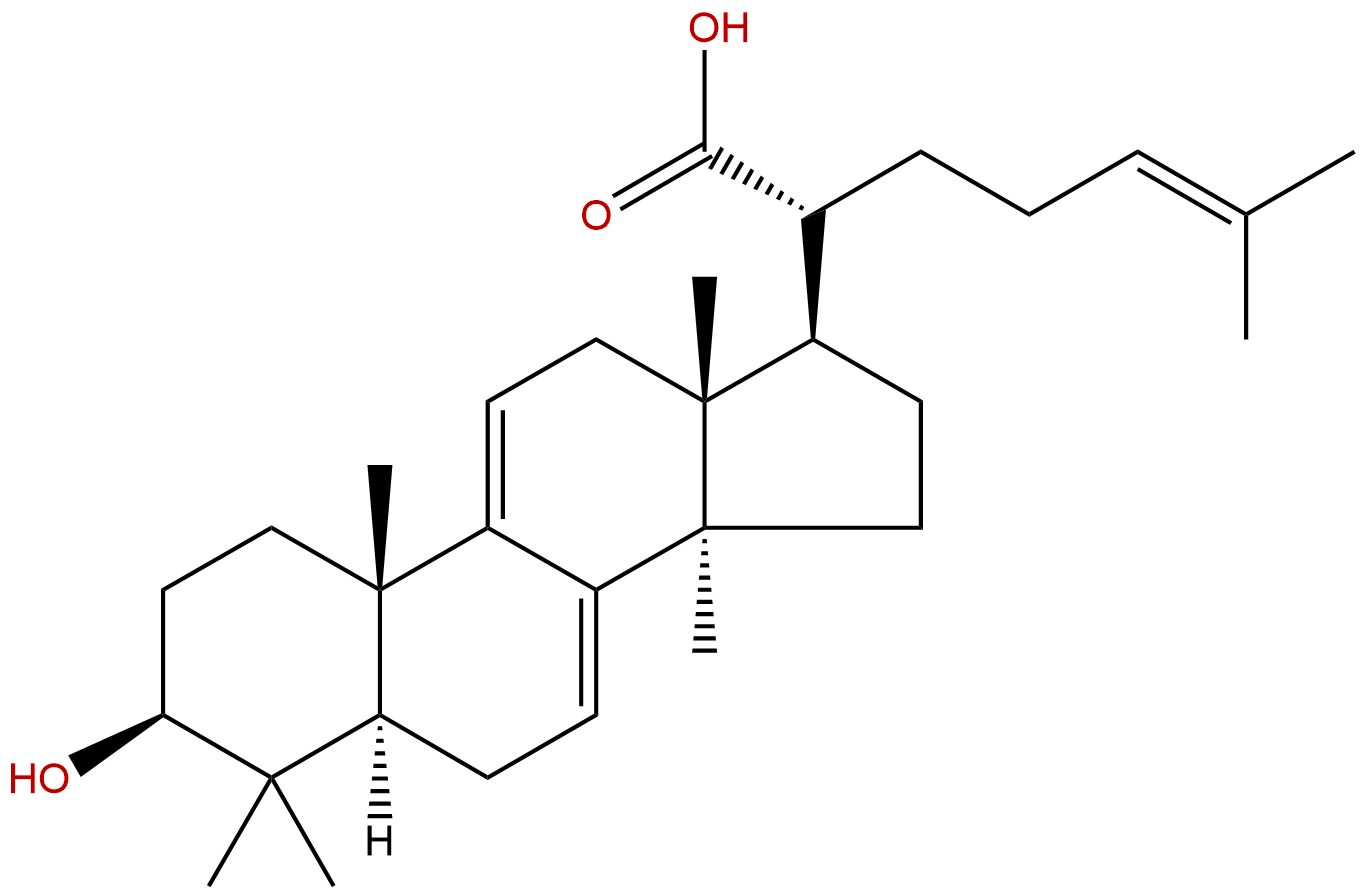
Dehydrotrametenolic acidCAS No.:29220-16-4
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1798 |
| Formula: | C30H46O3 |
| Mol Weight: | 454.695 |
Product name: Dehydrotrametenolic acid
Synonym name: 3-epidehydrotrametenolic acid
Catalogue No.: BP1798
Cas No.: 29220-16-4
Formula: C30H46O3
Mol Weight: 454.695
Botanical Source:
Physical Description: Powder
Type of Compound: Triterpenoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
The product could be supplied from milligrams to grams. Inquire for bulk scale.
We provide solution to improve the water-solubility of compounds, thereby facilitating the variety of activity tests and clinic uses.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
Dehydrotrametenolic acid is a promising candidate for a new type of insulin-sensitizing drug; it can be a potential anticancer agent against H-ras transformed tumor.
References:
Life Sci. 2006 Jan 2;78(6):607-13.
Dehydrotrametenolic acid selectively inhibits the growth of H-ras transformed rat2 cells and induces apoptosis through caspase-3 pathway.
The screening of natural products that preferentially inhibit growth of H-ras transformed rat2 cells vs. rat2 cells was performed to identify H-ras specific growth inhibitor.
METHODS AND RESULTS:
A lanostane-type triterpene acid, Dehydrotrametenolic acid (3beta-hydroxylanosta-7,9(11),24-trien-21-oic acid), was isolated from the sclerotium of Poria cocos (Polyporaceae). Dehydrotrametenolic acid selectively inhibited the growth of H-ras transformed cells with a GI(50) value of 40 microM. FACS analysis indicated that the compound exerted its anti-proliferation effects through cell cycle arrest at G2/M phase and accumulation of sub-G1 population. Dehydrotrametenolic acid-induced apoptosis was further confirmed with chromosomal DNA fragmentation, caspase-3 activation, and degradation of PARP and Lamin A/C degradation. The compound also regulated the expression of H-ras, Akt and Erk, which are the downstream proteins of H-ras signaling pathways.
CONCLUSIONS:
The results suggest that Dehydrotrametenolic acid can be a potential anticancer agent against H-ras transformed tumor.
Biol Pharm Bull. 2002 Jan;25(1):81-6.
Dehydrotrametenolic acid induces preadipocyte differentiation and sensitizes animal models of noninsulin-dependent diabetes mellitus to insulin.
We recently discovered that the triterpene acid compound Dehydrotrametenolic acid promotes adipocyte differentiation in vitro and acts as an insulin sensitizer in vivo. This natural product has been isolated from dried sclerotia of Poria cocos WOLF (Polyporaceae), a well-known traditional Chinese medicinal plant.
METHODS AND RESULTS:
We examined the effects of Dehydrotrametenolic acid on plasma glucose concentration in obese hyperglycemic db/db mice. Dehydrotrametenolic acid can reduce hyperglycemia in mouse models of noninsulin-dependent diabetes mellitus (NIDDM) and act as an insulin sensitizer as indicated by the results of the glucose tolerance test. These terpenoids and thiazolidine type of antidiabetic agents such as Ciglitazone, although structurally unrelated, share many biological activities: both induce adipose conversion, activate peroxisome proliferator-activated receptor gamma (PPAR gamma) in vitro, and reduce hyperglycemia in animal models of NIDDM. Dehydrotrametenolic acid is a promising candidate for a new type of insulin-sensitizing drug.
CONCLUSIONS:
This finding is very important for the development of insulin sensitizers that are not of the thiazolidine type.