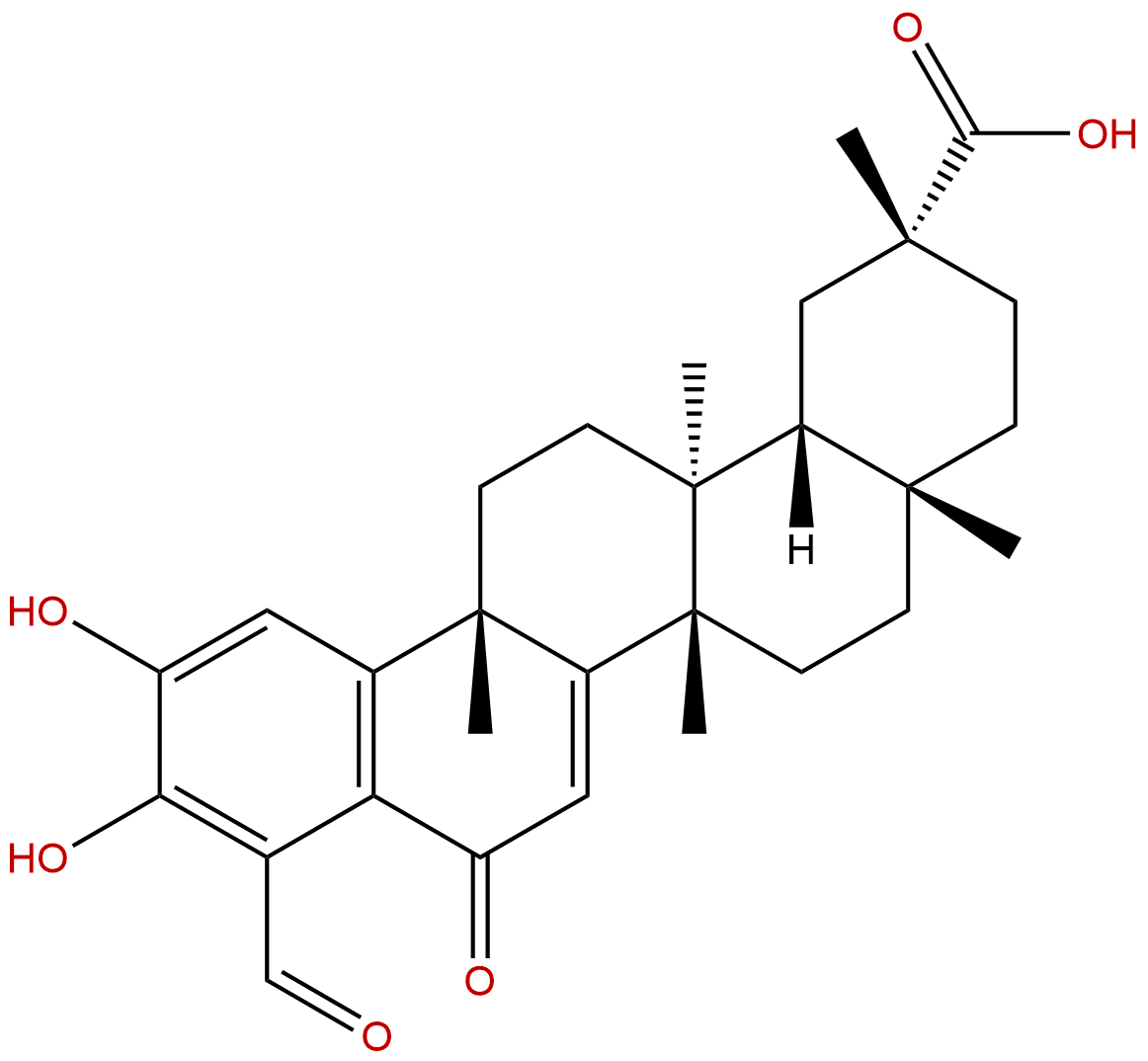
DemethylzeylasteralCAS No.:107316-88-1
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0477 |
| Formula: | C29H36O6 |
| Mol Weight: | 480.601 |
Synonym name:
Catalogue No.: BP0477
Cas No.: 107316-88-1
Formula: C29H36O6
Mol Weight: 480.601
Botanical Source: Kokoona zeylanica
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Description:
Demethylzeylasteral exhibits strong inhibition towards UDP-glucuronosyltransferase (UGT) 1A6 and 2B7, and the inhibition kinetic parameters (Ki) are calculated to be 0.6 uM and 17.3 uM for UGT1A6 and UGT2B7, respectively. Demethylzeylasteral has antimicrobial, strong immunosuppressive, and antifertility activities; it concentration-dependently and in a partially reversible manner can inhibit the Ca(2+) current in spermatogenic cells with an IC(50) of 8.8 microg/ml, and it also can inhibit significantly the sperm acrosome reaction initiated by progesterone.
References:
Eur J Drug Metab Pharmacokinet. 2014 Jun;39(2):99-102.
Demethylzeylasteral exhibits dose-dependent inhibitory behaviour towards estradiol glucuronidation.
The disturbance of estradiol level might induce the occurence of some diseases, including cancer. Estradiol is mainly metabolized through the conjugation reactions, including the sulfation and glucuronidation reactions. The present study tried to evaluate the inhibition of estradiol glucuronidation by the major ingredients of Tripterygium wilfordii Hook F. Demethylzeylasteral.
METHODS AND RESULTS:
Selective ion monitoring at negative ion mode ([M⁺ H⁻] = 447) was employed to monitor the two glucuronides of estradiol. The reaction rate was determined through comparison of peak area of these two glucuronides. Lineweaver-Burk plot and Dixon plot were utilized to determine the inhibition kinetic type, and the inhibition kinetic parameters (K i) were calculated using the second plot. Competitive inhibition of Demethylzeylasteral towards the formation of two glucuronides of estradiol was demonstrated, and the K i values were calculated to be 453.3 and 110.9 μM, respectively.
CONCLUSIONS:
All these results will remind us the risk of elevated serum concentrations of estradiol due to the inhibition of estradiol glucuronidation by Demethylzeylasteral.
Eur J Pharmacol. 2003 Mar 7;464(1):9-15.
Effects of demethylzeylasteral and celastrol on spermatogenic cell Ca2+ channels and progesterone-induced sperm acrosome reaction.
The male antifertility effect of a water-chloroform extract of Tripterygium wilfordii Hook. f. (GTW) and several monomers isolated from GTW has attracted worldwide interest.
METHODS AND RESULTS:
In the present study, the effects of two isolated monomers from GTW, Demethylzeylasteral and celastrol, on the Ca(2+) channels in mouse spermatogenic cells and on the sperm acrosome reaction were investigated by whole-cell patch-clamp recording and chlortetracycline staining methods, respectively. The results showed that Demethylzeylasteral concentration-dependently and in a partially reversible manner inhibited the Ca(2+) current in spermatogenic cells with an IC(50) of 8.8 microg/ml. Celastrol decreased the Ca(2+) current in the cells time-dependently and irreversibly. The changes in the activation and inactivation time constants of Ca(2+) currents after application of these two compounds were also examined. Demethylzeylasteral increased both activation and inactivation time constants of Ca(2+) currents, and celastrol had no significant effect on them. The two compounds also inhibited significantly the sperm acrosome reaction initiated by progesterone.
CONCLUSIONS:
These data suggest that inhibition of Ca(2+) currents could be responsible for the antifertility activity of these compounds.
Planta Med. 2005 Apr;71(4):313-9.
Antimicrobial activity of 6-oxophenolic triterpenoids. Mode of action against Bacillus subtilis.
Zeylasteral and Demethylzeylasteral are 6-oxophenolic triterpenoids isolated from the root of Maytenus blepharodes, which have antimicrobial activity against Gram-positive bacteria and the yeast Candida albicans.
METHODS AND RESULTS:
The time-kill curves for zeylasteral and Demethylzeylasteral at concentrations twice their MICs, against Bacillus subtilis showed that the colony forming units were reduced in 3-log10 and > 4-log10 respectively. This reduction was dependent on inoculum size and the growth phase of cells, and was greater when the compounds were incorporated in the exponential phase, indicating a bacteriolytic effect. Treatment with both agents, particularly with zeylasteral (20 microg/mL) caused a reduction of optical density at 550 nm. With regard to the synthesis of DNA, RNA, protein and cell wall, the compounds exhibited the fastest inhibition against cell wall synthesis.
CONCLUSIONS:
Thus, the predisposition to lysis, the morphological changes seen by microscopy, and the complete inhibition in the incorporation the N-acetyl-d-[1 - 14C]glucosamine, suggest that the phenolic compounds compromise the cell wall synthesis and/or cytoplasmic membrane.
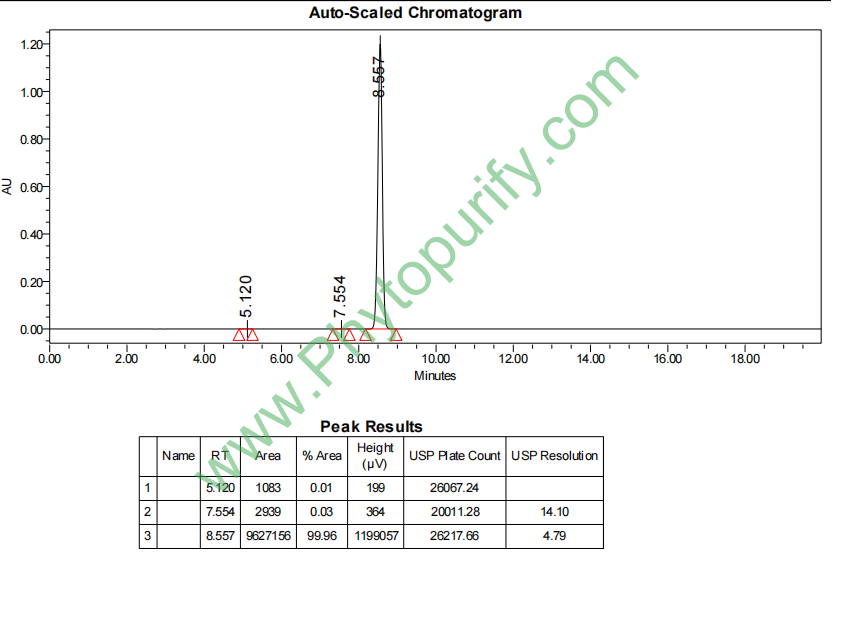
HPLC of Demethylzeylasteral