
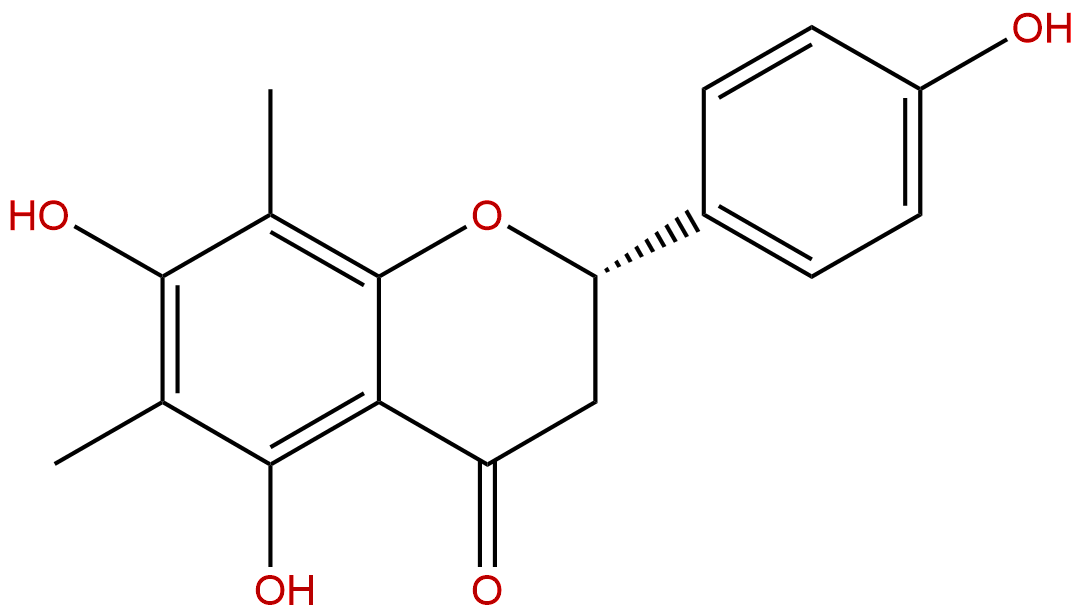
FarrerolCAS No.:24211-30-1
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0584 |
| Formula: | C17H16O5 |
| Mol Weight: | 300.31 |
Synonym name:
Catalogue No.: BP0584
Cas No.: 24211-30-1
Formula: C17H16O5
Mol Weight: 300.31
Botanical Source: the leaves of Rhododendron farrerae and other Rhododendron spp., from Cyrtomium spp., Phormium tenax and Angophora spp.
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Description:
Farrerol has antioxidative, anti-bacterial, anti-inflammatory, antiangiogenic activities, it is a potential candidate for the intervention of endothelial-injury-associated cardiovascular diseases. Farrerol inactivates KEAP-1 or activates the Akt, p38 and ERK to facilitate the release of Nrf2 from Keap1 and subsequent reduces the intracellular production of reactive oxygen species via the induction of HO-1 expression. Farrerol can inhibit angiogenesis through down regulation of Akt/mTOR, Erk and Jak2/Stat3 signal pathway, and can inhibit IL-1β-induced inflammatory responses in osteoarthritis chondrocytes by blocking PI3K/Akt/NF-κB signaling pathway.
References:
Chem Biol Interact. 2015 Jun 22.
The antioxidative potential of farrerol occurs via the activation of Nrf2 mediated HO-1 signaling in RAW 264.7 cells.
Farrerol, (S)-2,3-dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-6,8-dimethyl-4-benzopyrone, isolated from rhododendron, has been shown to have antioxidative potential, but the molecular mechanism underlying this activity remains unclear. The inducible expression of heme oxygenase-1 (HO-1), a potent antioxidative and cytoprotective enzyme, is known to play an important role in cytoprotection in a variety of pathological models.
METHODS AND RESULTS:
In this study, we evaluated the antioxidative potential of Farrerol against oxidative damage and investigated its antioxidative mechanism in RAW 264.7 cells. The molecular mechanism underlying the cytoprotective function of Farrerol was determined by analyzing intracellular signaling pathways, transcriptional activation and the inhibitory effect of HO-1 on ROS production. Farrerol induced antioxidant enzymes mRNA expression, HO-1 protein expression and nuclear translocation of NF-E2-related factor 2 in RAW 264.7 macrophage cells. Farrerol down-regulated the expression of the Keap1 protein and the thiol reducing agents attenuated Farrerol-induced HO-1 expression. Further investigation utilizing Western blotting and specific inhibitors of Akt, p38, JNK and ERK demonstrated that Akt, p38, and ERK axis of signaling pathway mediates HO-1 expression. Moreover, tert-butyl hydroperoxide (t-BHP)-induced oxidative damage was ameliorated by Farreroltreatment in a dose-dependent manner, which was abolished by Akt, p38, ERK and HO-1 inhibitors (Snpp). It is hence likely that Farrerol inactivated KEAP-1 or activated the Akt, p38 and ERK to facilitate the release of Nrf2 from Keap1 and subsequent reduced the intracellular production of reactive oxygen species via the induction of HO-1 expression.
CONCLUSIONS:
These results support the central role of HO-1 in the cytoprotective effect of Farrerol.
Microb Pathog. 2013 Dec;65:1-6.
Farrerol regulates antimicrobial peptide expression and reduces Staphylococcus aureus internalization into bovine mammary epithelial cells.
Mastitis, defined as inflammation of the mammary gland, is an infectious disease with a major economic influence on dairy industry. Staphylococcus aureus is a common gram-positive pathogen that frequently causes subclinical, chronic infection of the mammary gland in dairy cows. Farrerol, a traditional Chinese medicine isolated from rhododendron, has been shown to have anti-bacterial activity. However, the effect of Farrerol on S. aureus infection in mammary epithelium has not been studied in detail. The aim of this study was to investigate the effect of Farrerol on the invasion of bovine mammary epithelial cells (bMEC) by S. aureus.
METHODS AND RESULTS:
The expression of antimicrobial peptide genes by bMEC were assessed in the presence or absence of S. aureus infection. Our results demonstrated that Farrerol (4-16 μg/ml) reduced > 55% the internalization of S. aureus into bMEC. We also found that Farrerol was able to down-regulate the mRNA expression of tracheal antimicrobial peptide (TAP) and bovine neutrophil β-defensin 5 (BNBD5) in bMEC infected with S. aureus. The Nitric oxide (NO) production of bMEC after S. aureus stimulation was decreased by Farrerol treatment. Furthermore, Farrerol treatment suppressed S. aureus-induced NF-κB activation in bMEC.
CONCLUSIONS:
These results demonstrated that Farrerol modulated TAP and BNBD5 gene expression in mammary gland, enhances bMEC defense against S. aureus infection and could be useful in protection against bovine mastitis.
Eur J Pharmacol. 2015 Oct 5;764:443-7.
Anti-inflammatory effects of farrerol on IL-1β-stimulated human osteoarthritis chondrocytes.
The present study aimed to investigate the anti-inflammatory effects and the underlying molecular mechanism of Farrerol on IL-1β-stimulated human osteoarthritis chondrocytes.
METHODS AND RESULTS:
Chondrocytes were pretreated with Farrerol 1h before IL-1β stimulation. The effects of Farrerol on NO and PGE2 production were tested by Griess reagent and ELISA. The effects of Farrerol on COX-2, iNOS, Akt, phosphorylated Akt, and NF-κB activation were measured by western blot analysis. The results showed that Farrerol remarkably inhibited IL-1β-induced NO and PGE2 production, as well as COX-2 and iNOS expression. Farrerol also inhibited IL-1β-induced NF-κB activation. Furthermore, Farrerol significantly inhibited IL-1β-induced phosphorylation of PI3K and Akt.
CONCLUSIONS:
In conclusion, these results indicated that Farrerol inhibited IL-1β-induced inflammatory responses in osteoarthritis chondrocytes by blocking PI3K/Akt/NF-κB signaling pathway.