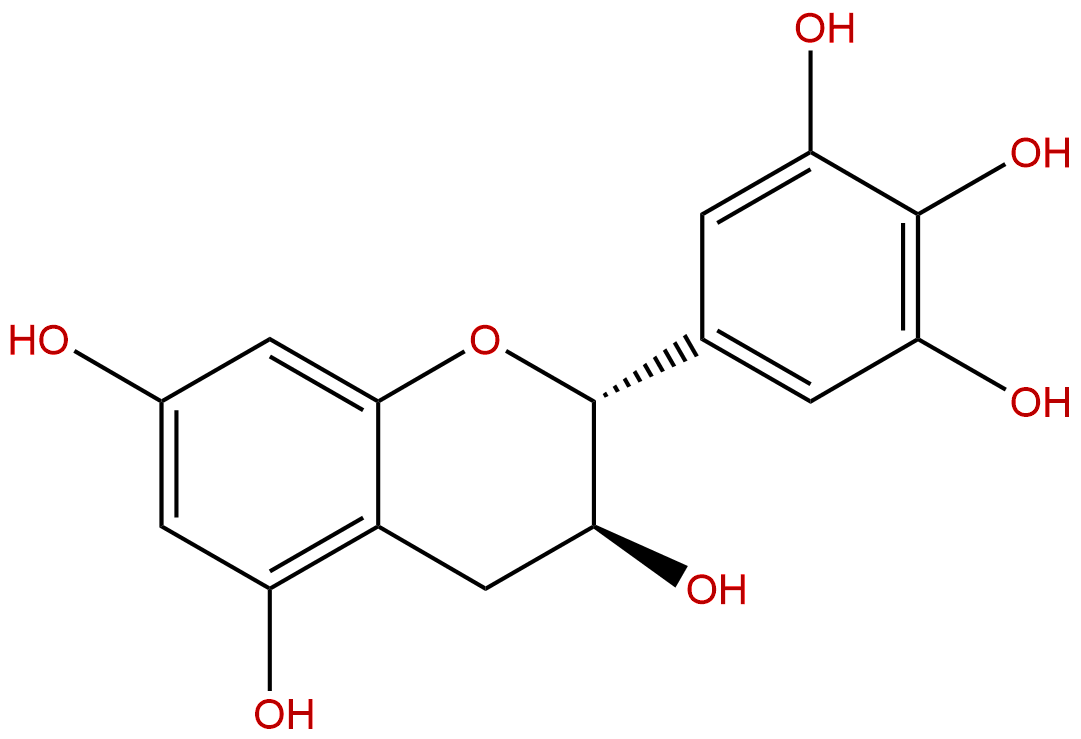
(+)-GallocatechinCAS No.:970-73-0
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1779 |
| Formula: | C15H14O7 |
| Mol Weight: | 306.27 |
Product name: (+)-Gallocatechin
Synonym name:
Catalogue No.: BP1779
Cas No.: 970-73-0
Formula: C15H14O7
Mol Weight: 306.27
Botanical Source:
Physical Description: White powder
Type of Compound: Flavonoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
The product could be supplied from milligrams to grams. Inquire for bulk scale.
We provide solution to improve the water-solubility of compounds, thereby facilitating the variety of activity tests and clinic uses.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
(+)-Gallocatechin as a bio-antimutagenic compound against UV-induced mutation in Escherichia coli. It is potent in scavenging Fremy’s salt, a synthetic free radical, it possesses antioxidant capacities that is higher or comparable to that of ascorbic acid or Trolox.
References:
Phytochemistry. 1994 Jul;36(4):1027-9.
Identification of (+)-gallocatechin as a bio-antimutagenic compound in Psidium guava leaves.[Pubmed: 7765204]
From the MeOH-extract of guava leaves, (+)-Gallocatechin was isolated as a bio-antimutagenic compound against UV-induced mutation in Escherichia coli. This strengthens the evidence that phenolic compounds require three neighbouring-OH groups in order to possess this activity.
Eur Food Res. Technol., 2004, 219(6):605-13.
Antioxidant gallocatechins, dimeric and trimeric proanthocyanidins from sea buckthorn (Hippophaë rhamnoides) pomace[Reference: WebLink]
Residues such as peels and seeds that result from fruit juice production may contain substantial amounts of valuable natural antioxidants.
METHODS AND RESULTS:
In order to isolate the potential antioxidants gallocatechins and proanthocyanidins, pomace from sea buckthorn (Hippophaë rhamnoides) berries was extracted by acetone-water (75:25, v/v) and fractionated by Sephadex LH-20 gel chromatography and semipreparative HPLC. The structures of the monomeric flavan-3-ols isolated, (+)-Gallocatechin and (−)-epigallocatechin, were elucidated by electrospray mass spectrometry (ESI-MS) and 1H and 13C NMR spectroscopy. Five dimeric proanthocyanidins were identified by HPLC-ESI-MS(-MS) and by acid-catalyzed cleavage in the presence of phloroglucinol. Moreover, nine trimeric proanthocyanidins were tentatively identified by HPLC-ESI-MS(-MS) in the Sephadex fractions of sea buckthorn pomace. The isolated flavan-3-ols and proanthocyanidins were potent in scavenging Fremy’s salt, a synthetic free radical.
CONCLUSIONS:
They possessed antioxidant capacities that were higher or comparable to that of ascorbic acid or Trolox. On comparing the antioxidant capacities of monomeric flavan-3-ols and dimeric proanthocyanidins, no significant influence from the degree of polymerization (DP) was observed.
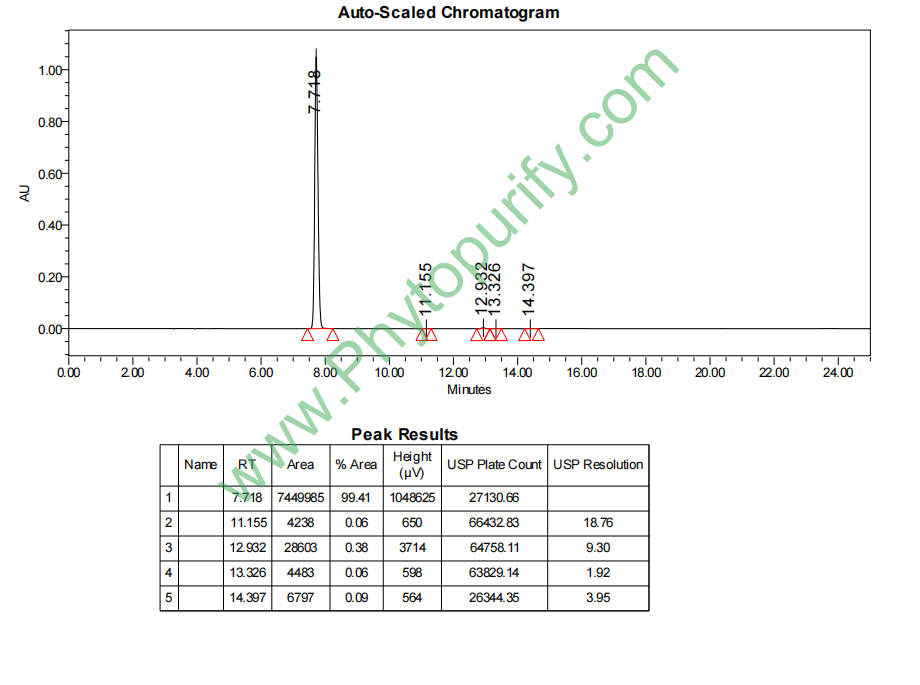
HPLC of (+)-Gallocatechin