Hederacoside CCAS No.:14216-03-6
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0708 |
| Formula: | C59H96O26 |
| Mol Weight: | 1221.39 |
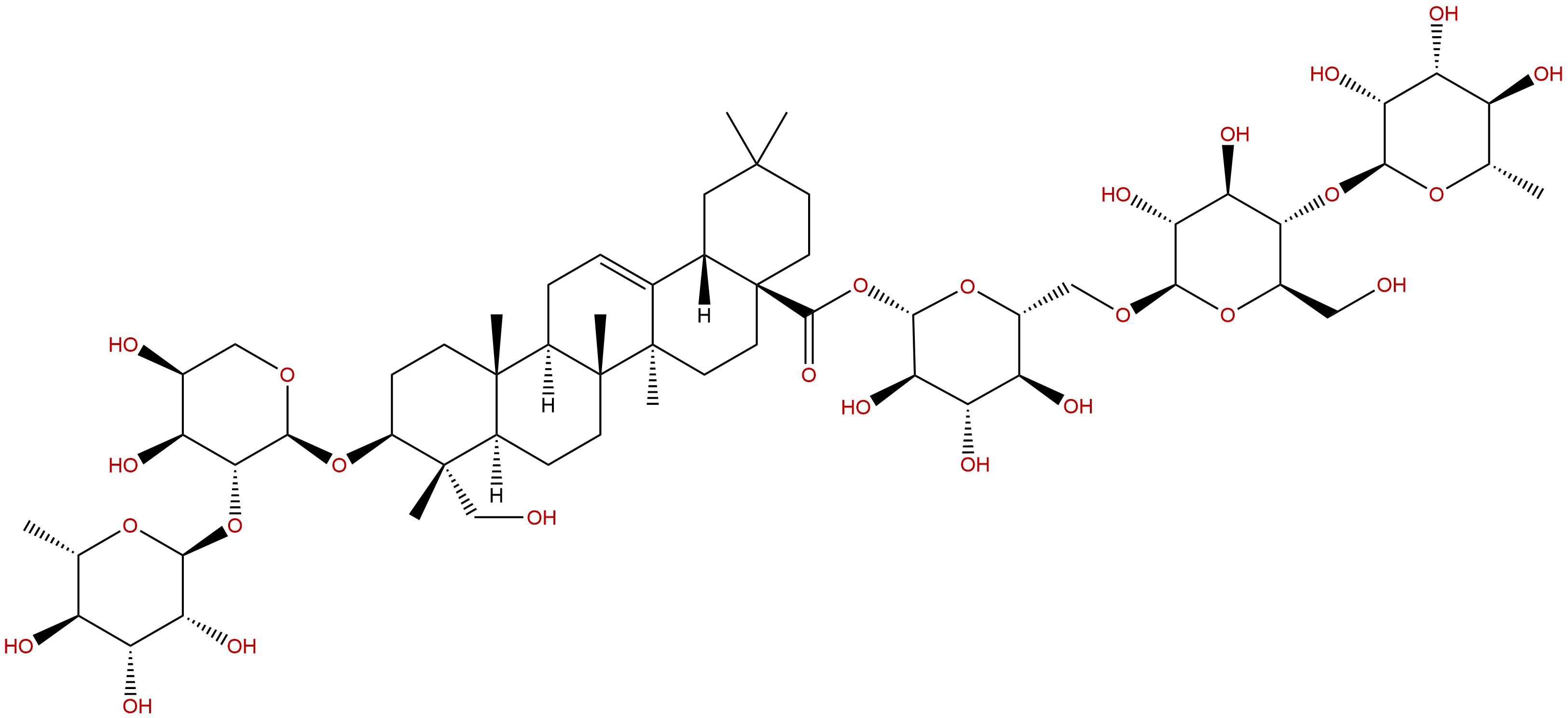
Product Name: Hederacoside C
Synonym name: HDC; Hederasaponin C; Hederacaucaside D
Cas No.: 14216-03-6
Formula: C59H96O26
Mol Weight: 1221.39
Botanical Source: Hedera helix; Chinese ivy; Hedera nepalensis K,Koch var.sinensis (Tobl.) Rehd
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams, up to kilograms.
Inquire for bulk scale.
Reference
Pharmacokinetics of hederacoside C, an active ingredient in AG NPP709, in rats.
Xenobiotica. 2013 Nov;43(11):985-92.
Abstract
1. Hederacoside C (HDC) is one of the active ingredients in Hedera helix leaf extract (Ivy Ex.) and AG NPP709, a new botanical drug to treat acute respiratory infection and chronic inflammatory bronchitis. However, information regarding its pharmacokinetic properties remains limited. 2. Here, we report the pharmacokinetics of HDC in rats after intravenous administration of HDC (3, 12.5, and 25 mg/kg) and after oral administration of HDC, Ivy Ex., and AG NPP709 (equivalent to 12.5, 25, and 50 mg/kg HDC). 3. Linear pharmacokinetics of HDC were identified upon its intravenous administration at doses of 3-25 mg/kg. Intravenous administration of HDC results in relatively slow clearance (1.46-2.08 mL/min/kg) and a small volume of distribution at steady state (138-222 mL/kg), while oral administration results in a low absolute oral bioavailability (F) of 0.118-0.250%. The extremely low F of HDC may be due to poor absorption of HDC from the gastrointestinal (GI) tract and/or its decomposition therein. 4. The oral pharmacokinetics of HDC did not differ significantly among pure HDC, Ivy Ex., and AG NPP709.
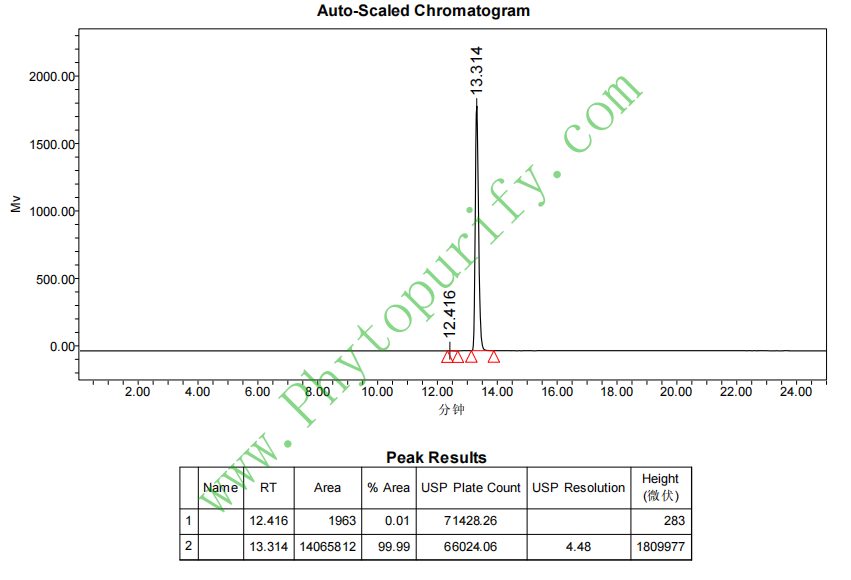
HPLC of Hederacoside C