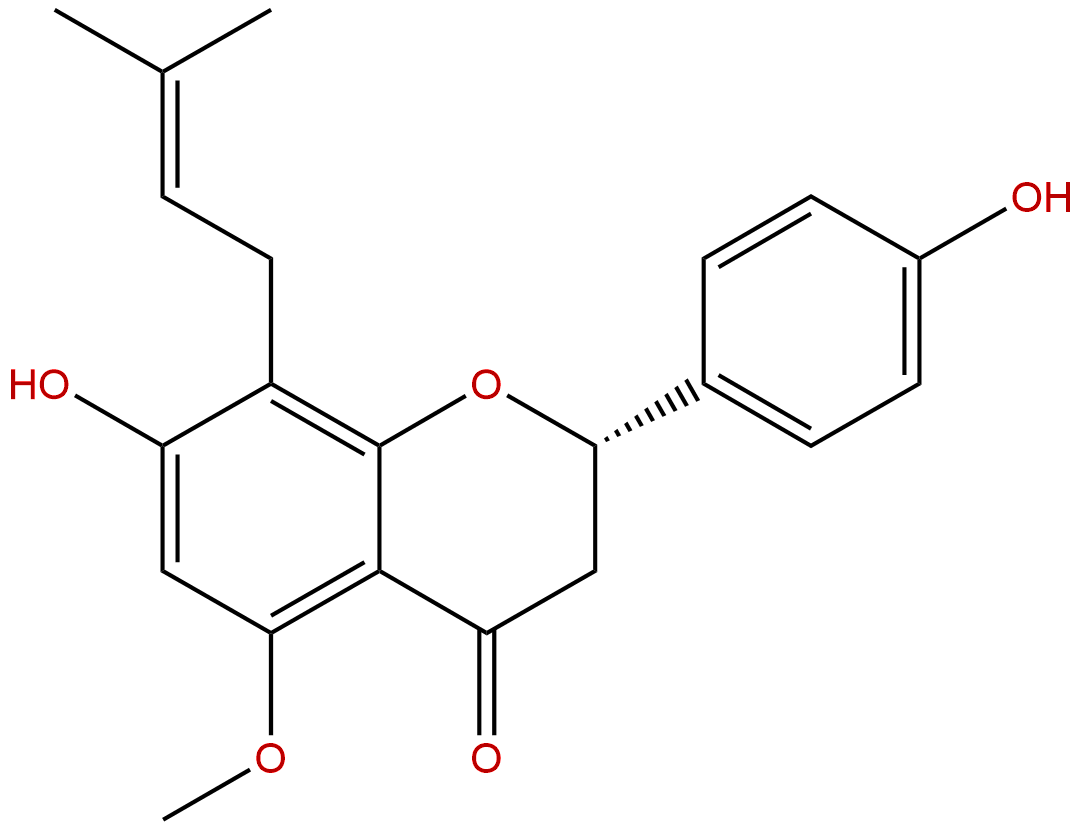
IsoxanthohumolCAS No.:70872-29-6
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0805 |
| Formula: | C21H22O5 |
| Mol Weight: | 354.402 |
Product name: Isoxanthohumol
Synonym name: 4′,7-Dihydroxy-5-methoxy-8-prenylflavanone; Humulol; 521-48-2
Catalogue No.: BP0805
Cas No.: 70872-29-6
Formula: C21H22O5
Mol Weight: 354.402
Botanical Source: Humulus lupulus Linn.
Physical Description:
Type of Compound: Flavonoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Isoxanthohumol is a polyphenol with antioxidant, anti-inflammatory, and antiangiogenic properties, seems to regulate in vivo vascular proliferation and stabilization and the EC-VSMC-inflammatory crosstalk. Isoxanthohumol shows an antiviral activity towards herpes viruses (HSV1 and HSV2) and bovine viral diarrhea virus (BVDV).
References:
Fitoterapia. 2015 Jun;103:71-82.
Isoxanthohumol - Biologically active hop flavonoid.
Isoxanthohumol (IXN), apart from xanthohumol (XN) and 8-prenylnaringenin (8PN), is one of the most important prenylflavonoids found in hops. Another natural source of this compound is a shrub Sophora flavescens, used in traditional Chinese medicine. Main dietary source of Isoxanthohumol is beer, and the compound is produced from XN during wort boiling.
METHODS AND RESULTS:
In the human body, the compound is O-demethylated to 8PN, the strongest known phytoestrogen. This process takes place in the liver and in the intestine, where it is mediated by local microflora. It has been reported in some studies that even though beer contains small amounts of hops and its preparations, these compounds may affect the functioning of the human body. Isoxanthohumolexhibits an antiproliferative activity against human cell lines typical for breast cancer (MCF-7), ovarian cancer (A-2780), prostate cancer (DU145 and PC-3), and colon cancer (HT-29 and SW620) cells. It strongly inhibits the activation of the following carcinogens: 2-amino-3-methylimidazol-[4,5-f]quinoline and aflatoxin B1 (AFB1) via human cytochrome P450 (CYP1A2). It also inhibits the production of prostate specific antigen (PSA). Isoxanthohumol significantly reduces the expression of transforming growth factor-β (TGF-β) in the case of invasive breast cancer MDA-MB-231. It interferes with JAK/STAT signaling pathway and inhibits the expression of pro1inflammatory genes in the monoblastic leukemia cell line (MonoMac6). It activates apoptosis in human umbilical vein endothelial cells (HUVEC) and human aortic smooth muscle cells (HASMCs). In addition, Isoxanthohumol shows an antiviral activity towards herpes viruses (HSV1 and HSV2) and bovine viral diarrhea virus (BVDV).
Biofactors. 2013 Nov-Dec;39(6):608-22.
Isoxanthohumol modulates angiogenesis and inflammation via vascular endothelial growth factor receptor, tumor necrosis factor alpha and nuclear factor kappa B pathways.
Angiogenesis and inflammation are becoming distinguished players in the pathogenesis of many heterogeneous diseases, such as diabetes, cardiovascular disease, and cancer. Therefore, it is crucial to study new compounds that are able to modulate these events. Isoxanthohumol (IXN) is a polyphenol with antioxidant, anti-inflammatory, and antiangiogenic properties.
METHODS AND RESULTS:
The aim of this study was to evaluate the effects of IXN on blood vessel proliferation and maturation and describe underlying molecular mechanisms in endothelial cells (ECs) and vascular smooth muscle cells (VSMCs). Angiogenic profile of IXN was analyzed by retinal angiogenesis at different time points. IXN modulation of angiogenic and inflammatory signaling pathways was evaluated by Western blotting on EC and VSMC cultures. IXN inhibited by 20% sprouting angiogenesis and decreased vascular coverage by mural cells up to 39%. IXN of 10 µM also decreased inflammatory signals, namely tumor necrosis factor alpha (TNF-α) (26 and 40%) and factor nuclear kappa B (24 and 42%) in human aortic smooth muscle cells (HASMCs) and human umbilical vein endothelial cells (HUVECs). Angiogenic regulators, including vascular endothelial growth factor receptor 2 (HUVEC, 55%), angiopoietins 1 (HUVEC, 39%; HASMC, 35%), angiopoietin 2 (HUVEC, 38%), and Tie2 (HUVEC, 56%) were also inhibited by 10 µM of IXN treatments. Akt activation was reduced by 47% in HUVEC-treated cells and Erk activation was also reduced by 52 and 69% upon IXN treatment of HUVEC and HASMC.
CONCLUSIONS:
IXN seems to regulate in vivo vascular proliferation and stabilization and the EC-VSMC-inflammatory crosstalk, leaving this molecule as an interesting nutritional player for angiogenesis and inflammation-related diseases.
Eur J Nutr. 2016 Feb;55(1):257-65.
Isoxanthohumol, a constituent of hop (Humulus lupulus L.), increases stress resistance in Caenorhabditis elegans dependent on the transcription factor DAF-16.
The flavanone Isoxanthohumol (IX) has gained attention as antioxidative and chemopreventive agent, but the molecular mechanism of action remains unclear. We investigated effects of this secondary plant compound in vivo using the model organism Caenorhabditis elegans.
METHODS AND RESULTS:
Adult C. elegans nematodes were incubated with Isoxanthohumol, and then, the stress resistance was analysed in the SYTOX assay; lifespan was monitored by touch-provoked movement method, the amount of reactive oxygen species (ROS) was measured in the DCF assay, and the nuclear localisation of the transcription factor DAF-16 was analysed by using a transgenic strain. By the use of a DAF-16 loss-of-function strain, we analysed whether the effects are dependent on DAF-16. Isoxanthohumol increases the resistance of the nematode against thermal stress. Additionally, a reduction in ROS in vivo was caused by Isoxanthohumol. Since the flavanone only has a marginal radical-scavenging capacity (TEAC assay), we suggest that Isoxanthohumolmediates its antioxidative effects indirectly via activation of DAF-16 (homologue to mammalian FOXO proteins). The nuclear translocation of this transcription factor is increased by Isoxanthohumol. In the DAF-16-mutated strain, the Isoxanthohumol-mediated increase in stress resistance was completely abolished; furthermore, an increased formation of ROS and a reduced lifespan was mediated by Isoxanthohumol.
CONCLUSIONS:
Isoxanthohumol or a bacterial metabolite of Isoxanthohumol causes antioxidative effects as well as an increased stress resistance in C. elegans via activation of DAF-16. The homologous pathway may have implications in the molecular mechanism of Isoxanthohumol in mammals.
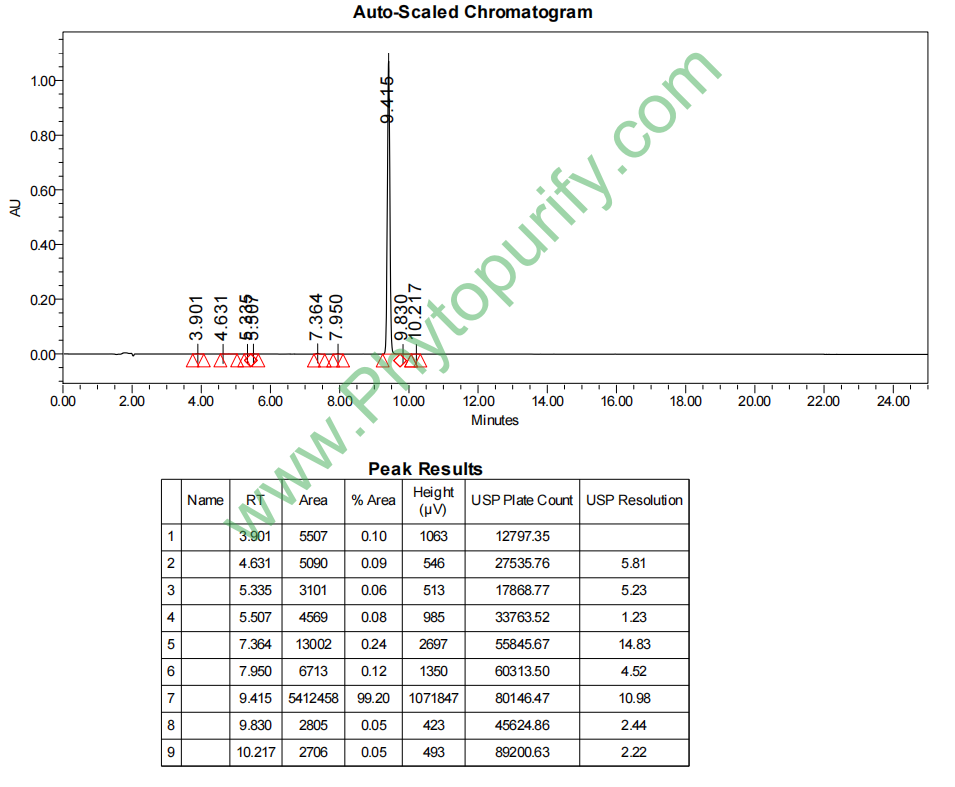
HPLC of Isoxanthohumol