
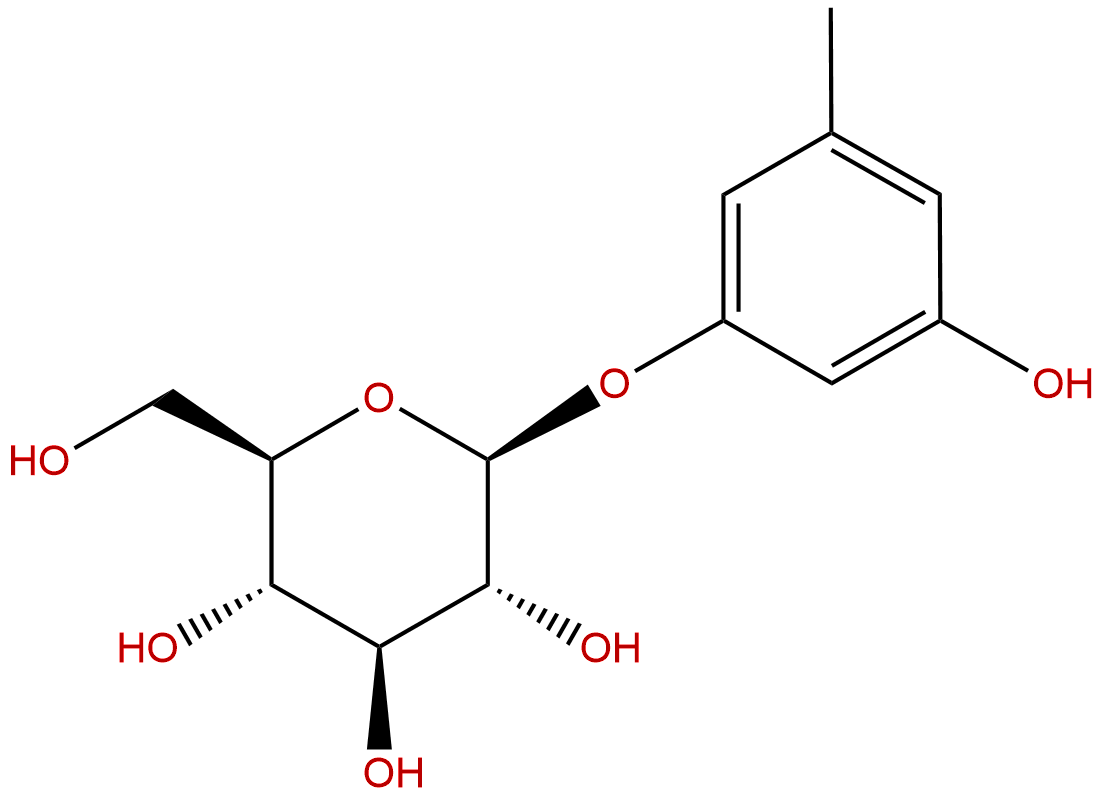
Orcinol glucosideCAS No.:21082-33-7
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1040 |
| Formula: | C13H18O7 |
| Mol Weight: | 286.28 |
Product name: Orcinol glucoside
Synonym name: Sakakin
Catalogue No.: BP1040
Cas No.: 21082-33-7
Formula: C13H18O7
Mol Weight: 286.28
Botanical Source: Curculigo orchioides Gaertn.
Physical Description:
Type of Compound: Phenols
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Orcinol glucoside(Sakakin) shows potent antioxidative and anxiolytic activities without sedative effects, it can improve depressive behaviour in chronic unpredictable mild stress (CUMS) rats by downregulating HPA axis hyperactivity and increasing BDNF expression and ERK1/2 phosphorylation in the hippocampus.
References:
Chem Pharm Bull (Tokyo). 2005 Aug;53(8):1065-7.
Antioxidative phenols and phenolic glycosides from Curculigo orchioides.
A new orcinol glucoside, orcinol-1-O-beta-D-apiofuranosyl-(1-->6)-beta-D-glucopyranoside (3), was isolated from the rhizomes of Curculigo orchioides GAERTN., together with seven known compounds: orcinol glucoside (1), orcinol-1-O-beta-D-glucopyranosyl-(1-->6)-beta-D-glucopyranoside (2), curculigoside (Sakakin,4), curculigoside B (5), curculigoside C (6), 2,6-dimethoxyl benzoic acid (7), and syringic acid (8).
METHODS AND RESULTS:
The structures of these compounds were elucidated using spectroscopic methods. The antioxidant activities of these isolated compounds were evaluated by colorimetric methods based on their scavenging effects on hydroxyl radicals and superoxide anion radicals, respectively.
CONCLUSIONS:
All the compounds showed potent antioxidative activities and the structure-activity relationship is discussed.
Eur. Neuropsychopharmacol. ,2014 ,24(1): 172-80.
Orcinol glucoside produces antidepressant effects by blocking the behavioural and neuronal deficits caused by chronic stress.
This study focused on the antidepressant potential of orcinol glucoside (Sakakin,OG) and its possible mechanisms of action.
METHODS AND RESULTS:
We established a depressed rat model using 3 consecutive weeks of chronic unpredictable mild stress (CUMS). The antidepressant-like effect of OG was revealed using the sucrose preference test, the open field test, the forced swimming test (FST), and the tail suspension test (TST). The activity of the hypothalamic-pituitary-adrenal (HPA) axis was evaluated by detecting the serum corticosterone (CORT) concentrations and mRNA expression of corticotrophin-releasing hormone (CRH) in the hypothalamus. The protein expression levels of brain-derived neurotrophic factor (BDNF) and total phosphorylated-ERK1/2 were detected by western blot. The results showed that OG treatment (1.5, 3, or 6mg/kg) alleviated the depression-like behaviour of rats under CUMS, as indicated by the increased sucrose preference and the decreased immobility in both the FST and TST, although the rearing frequency in the open field test increased only in the group that received the lowest dose (1.5mg/kg OG). Rats that received OG treatment exhibited reduced serum CORT levels and CRH mRNA expression in the hypothalamus, suggesting that the hyperactivity of the HPA axis in CUMS rats was reversed by OG treatment. Moreover, OG treatment upregulated the protein levels of BDNF and phosphorylated-ERK1/2 in the hippocampus, even above control levels.
CONCLUSIONS:
Our findings suggest that OG improved depressive behaviour in CUMS rats by downregulating HPA axis hyperactivity and increasing BDNF expression and ERK1/2 phosphorylation in the hippocampus.
HPLC of Orcinol glucoside
