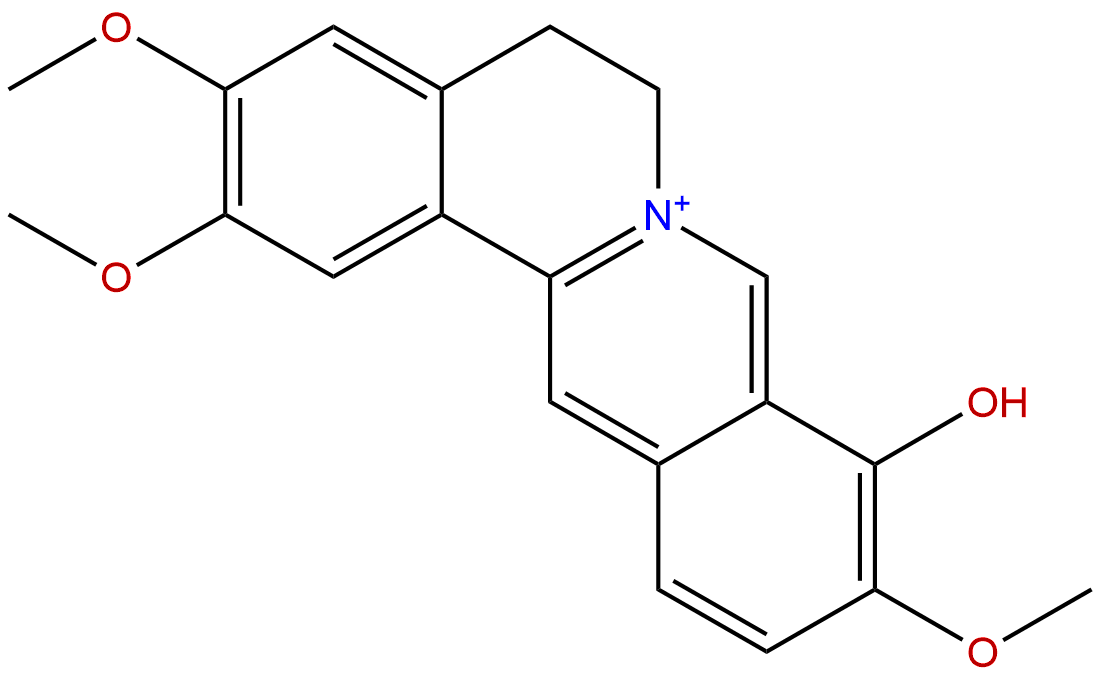
PalmatrubineCAS No.:16176-68-4
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1619 |
| Formula: | C20H20NO4+ |
| Mol Weight: | 338.382 |
Product name: Palmatrubine
Synonym name:
Catalogue No.: BP1619
Cas No.: 16176-68-4
Formula: C20H20NO4+
Mol Weight: 338.382
Botanical Source: Alkaloid from Fibraurea chloroleuca and Stephania glabra tubers (Menispermaceae)
Physical Description: Powder
Type of Compound: Alkaloids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
The product could be supplied from milligrams to grams. Inquire for bulk scale.
We provide solution to improve the water-solubility of compounds, thereby facilitating the variety of activity tests and clinic uses.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
Palmatrubine binds to double-stranded DNA most probably via an intercalating mode.
References:
Chem Biodivers. 2007 Feb;4(2):145-53.
DNA-binding affinities and sequence specificities of protoberberine alkaloids and their demethylated derivatives: a comparative study.
METHODS AND RESULTS:
Berberrubine (1a), jatrorubine (2a), and Palmatrubine (3a) have been chemically prepared by partial demethylation of berberine (1), jatrorrhizine (2), and palmatine (3), respectively. Their interactions with calf thymus (CT) DNA, poly(dA-dT)poly(dA-dT), poly(dG-dC)poly(dG-dC), and eight AT-rich 12-mer double-stranded DNAs have been investigated by means of competitive ethidium bromide (EB) displacement experiments. The results showed that DNA-binding affinities of these protoberberine alkaloids have been significantly improved by partial demethylation, and that all of these alkaloids have the preferable binding affinities with AT-rich DNA. Especially, the sequence specificities of DNA-binding of demethylated derivatives 1a, 2a, and Palmatrubine had changed to a certain extent when compared with the parent alkaloids 1, 2, and 3, respectively. The binding mode of these alkaloids was further confirmed by UV spectroscopic titration experiments.
CONCLUSIONS:
All the compounds bind to double-stranded DNA most probably via an intercalating mode.
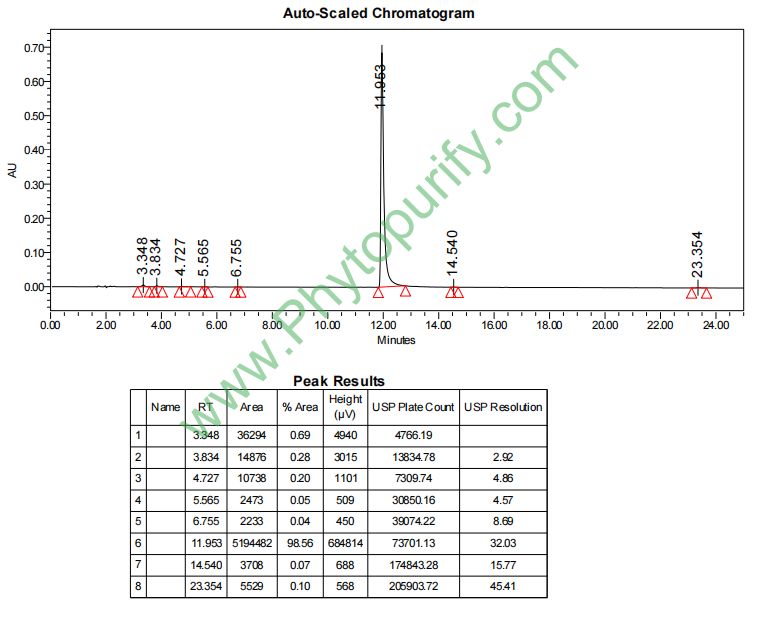
HPLC of Palmatrubine