
Tubuloside ACAS No.:112516-05-9 |
||||||||||
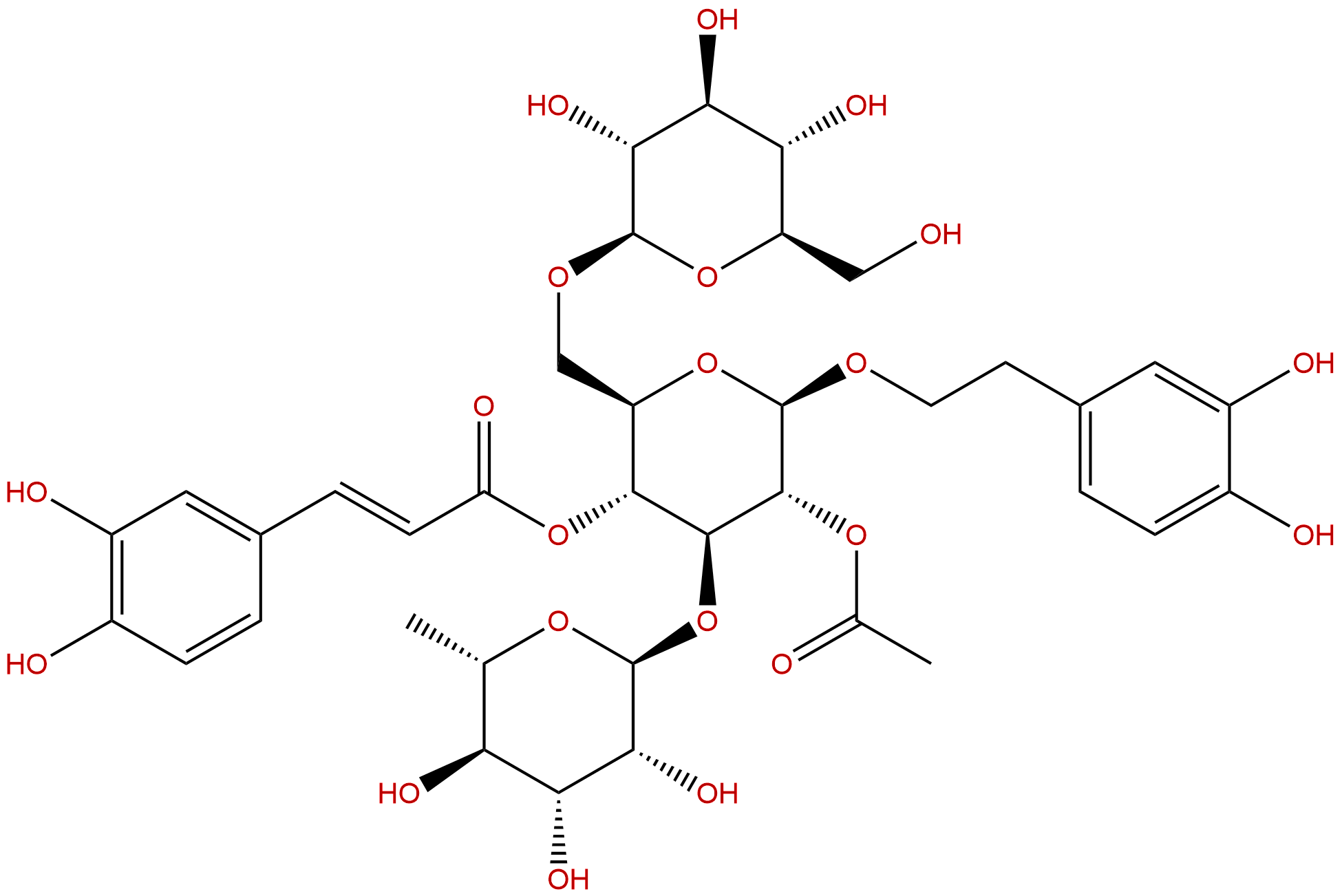 |
|
|
||||||||

| Catalogue No.: | BP1491 |
| Formula: | C37H48O21 |
| Mol Weight: | 828.77 |
| Botanical Source: | Cistanches herba |
Synonym name:
Catalogue No.: BP1491
Cas No.: 112516-05-9
Formula: C37H48O21
Mol Weight: 828.77
Botanical Source: Cistanche tubulosa, a parasitic plant growing on roots of Salvadora calotropis
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams. Inquire for bulk scale.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
Tubuloside A has anti-inflammatory, antioxidative and hepatoprotective activities. Tubuloside A shows stronger free radical scavenging activities than alpha-tocopherol on 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical and xanthine/xanthine oxidase (XOD) generated superoxide anion radical (O2-.).
References;
Plant Biotechnology 12(1), 46-54, 1995-04-01
Studies on the Tissue Culture of Cistanche Plants: I. Callus Induction and Shoot Differentiation from the Seed of Cistanche tubulosa Wight
Cistanche tubulosa Wight. is an important herbal medicine including such phenylethanoide glycosides as echinacoside (E), Tubuloside A (TA), acteoside (A) and 2′-acetylacteoside (2′AA), and is a holoparasitic plant distributed in a wide range from the North African to Chinese in and and semi arid regions.
METHODS AND RESULTS:
In this plant, seed germination occured without a host plant by the addition of gibberellic acid to the medium and the removal of the seed coat. Callus were induced on 1/2MS medium containing 2.5mg/l indol-3-acetic acid and 0.5mg/l kinetin, and with chilling treatment (at 4°C for 3 months) of the seeds. With this condition, a shoot differentiated from the callus in 6 weeks, but a parasitic root did not form. The shape of the vascular bundle of the shoot and that of the mother plant was slightly different.The largest value for phenylethanoide glycosides contained in the shoot was 10 fold that of the mother plant.
Eur J Pharmacol. 2000 Jul 14;400(1):137-44.
Inhibition of nitric oxide by phenylethanoids in activated macrophages.
Nitric oxide (NO) is one of the pro-inflammatory molecules. Some phenylethanoids have been previously shown to possess anti-inflammatory effects.
METHODS AND RESULTS:
Seven phenylethanoids from the stems of Cistanche deserticola, viz. isoacteoside, tubuloside B, acteoside, 2'-O-acetylacteoside, echinacoside, cistanoside A and Tubuloside A, were tested for their effect on NO radical generation by activated murine macrophages. At the concentration of 100-200 microM, all the phenylethanoids reduced (6.3-62.3%) nitrite accumulation in lipopolysaccharide (0.1 microgram/ml)-stimulated J774.1 cells. At 200 microM, they inhibited by 32.2-72.4% nitrite accumulation induced by lipopolysaccharide (0.1 microgram/ml)/interferon-gamma (100 U/ml) in mouse peritoneal exudate macrophages. However, these compounds did not affect the expression of inducible nitric oxide (iNOS) mRNA, the iNOS protein level, or the iNOS activity in lipopolysaccharide-stimulated J774.1 cells. Instead, they showed a clear scavenging effect (6.9-43.9%) at the low concentrations of 2-10 microM of about 12 microM nitrite generated from an NO donor, 1-propanamine-3-hydroxy-2-nitroso-1-propylhydrazino (PAPA NONOate).
CONCLUSIONS:
These results indicate that the phenylethanoids have NO radical-scavenging activity, which possibly contributes to their anti-inflammatory effects.