
Isocucurbitacin BCAS No.:17278-28-3 |
||||||||||
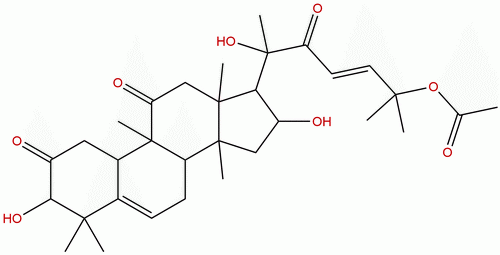 |
|
|
||||||||

| Catalogue No.: | BP3973 |
| Formula: | C32H46O8 |
| Mol Weight: | 558.712 |
| Botanical Source: | Cucumis melo L. |
Product name: Isocucurbitacin B
Synonym name:
Catalogue No.: BP3973
Cas No.: 17278-28-3
Formula: C32H46O8
Mol Weight: 558.712
Botanical Source: Cucumis melo L.
Physical Description:
Type of Compound:
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
The product could be supplied from milligrams to grams
Inquire for bulk scale.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
Isocucurbitacin B has significantly activities against HeLa and HT-29 human cancer cells with IC50 values ranging from 0.93 to 9.73uM.
References:
J Ethnopharmacol. 2015 Jul 1;169:18-23.
Bioassay-guided isolation and identification of cytotoxic compounds from Bolbostemma paniculatum.
Four cucurbitacine triterpenoid sapogenins, Isocucurbitacin B(1), 23,24-dihydroIsocucurbitacin B(2), cucurbitacin E(3), 23,24-dihydrocucurbitacin E(4), and 11 triterpenoid saponins, tubeimosideI(5), tubeimoside III(6), tubeimoside V(7), dexylosyltubeimoside III(8), lobatoside C(9), tubeimoside A(10), tumeimoside B(11), lobatoside A(12), tubeimoside C(13), tubeimoside IV(14), 7β,18,20,26-tetrahydroxy-(20S)-dammar-24E-en-3-O-α-L-(4-acetyl)arabinopyranosyl-(1→2)-β-D-glucopyranoside(15) were isolated from the active EtOAc and n-BuOH extracts.
METHODS AND RESULTS:
Of them, compounds 2, 4, 9 and 12 were firstly isolated from the Bolbostemma genus. MTT assay revealed that compounds 1, 3 and 4 had significantly activities against HeLa and HT-29 human cancer cells with IC50 values ranging from 0.93 to 9.73μM. It is worth mentioning that compound 4׳s activities against the two cell lines are 12- and 8-fold that of the positive control drug (5-Fu). Whereas, the cyclic bisdesmosides 5-9 exerted significantly activities on BGC-823, HeLa, HT-29 and MCF-7 cancer cells with IC50 values ranging from 1.30 to 15.64μM. And 6׳s activities against the four cell lines are 6-, 3-, 10- and 16-fold that of 5-Fu and 8׳s activities against the four cell lines are 5-, 3-, 14- and 9-fold that of 5-Fu.
CONCLUSIONS:
The cytotoxic activity of the bulbs of B. paniculatum is mainly ascribable to cucurbitacine triterpenoid sapogenins (1-4) and the cyclic bisdesmosides (5-9). The cyclic bisdesmosides are the main anti-cancer active compounds of B. paniculatum. The above results provide scientific evidence to support, to some extent, the ethnomedicinal use of B. paniculatum as anticancer remedies in traditional Chinese medicine.