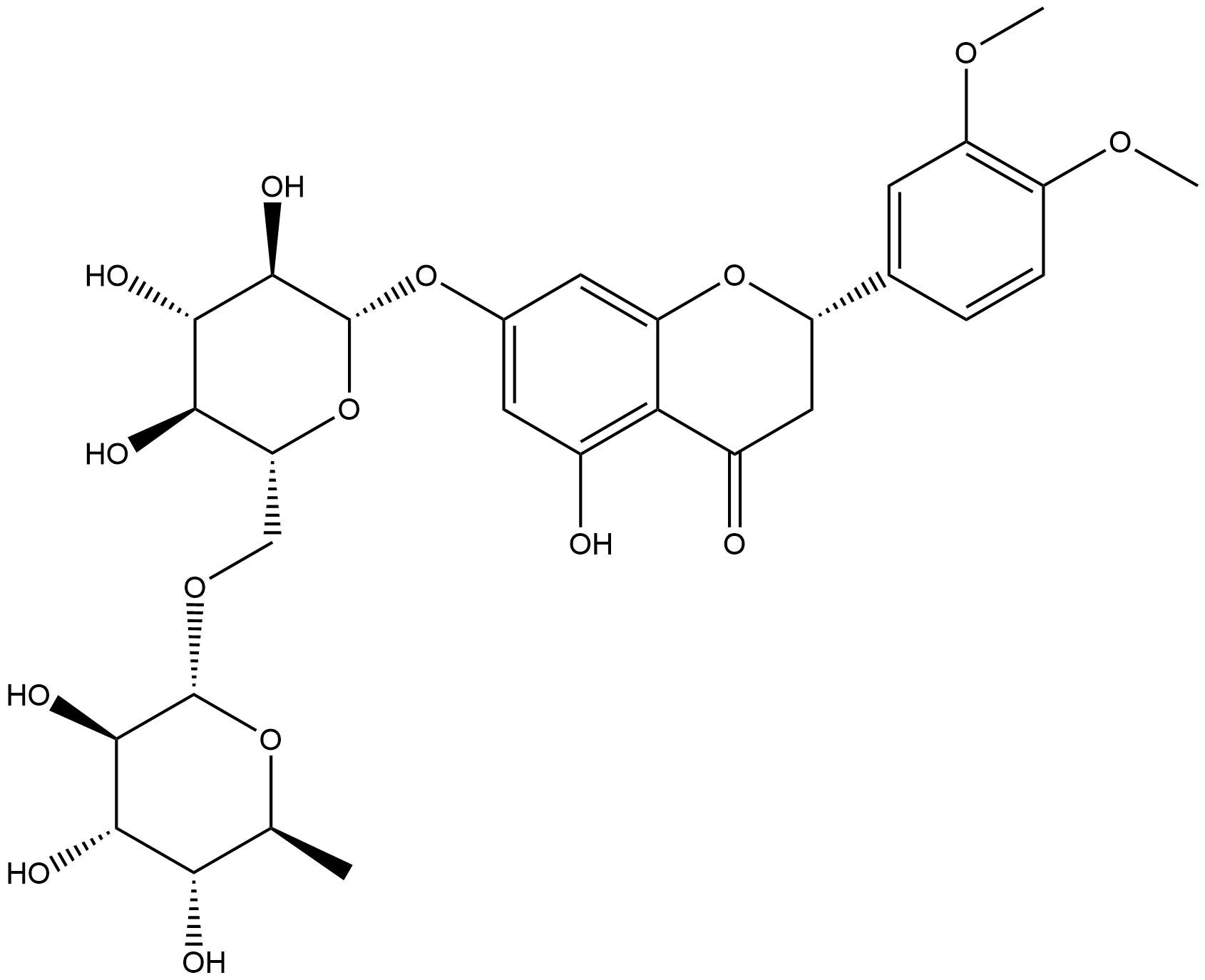
Methyl HesperidinCAS No.:11013-97-1 |
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0937 |
| Formula: | C29H36O15 |
| Mol Weight: | 624.592 |
| Botanical Source: | Aurantii fructus immaturus |
Synonym name:
Catalogue No.: BP0937
Cas No.: 11013-97-1
Formula: C29H36O15
Mol Weight: 624.592
Botanical Source:
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Description:
Methyl hesperidin has potentiating effect on coronary vasodilation induced by adenosine or related compounds, It caused inhibition of nitrendipine transport in the ileum and jejunum, but not in the duodenum. Methyl hesperidin exerts no obvious toxic effects in mice of either sex when administered at a level as high as 5.0% in the diet.
References:
Drug Metabol Drug Interact. 2008;23(3-4):299-310.
Influence of some bioflavonoids on the transport of nitrendipine.
Flavonoids form a large class of phenolic substances widely distributed in nature and exhibit several biological effects. P-glycoprotein is part of a large family of efflux transporters found in the gut, gonads and other organs.
METHODS AND RESULTS:
Male albino rats were used for this study. The whole small intestine was flushed with 50 ml of ice-cold saline after sacrificing the animal with an overdose of pentobarbital. The small intestine was isolated and divided into duodenum, jejunum and ileum. Each segment was everted, a 5-cm long sac was prepared, 1 ml of nitrendipine solution was introduced into the everted sac (serosal side), and both ends of the sac were ligated tightly. The sac containing nitrendipine solution was immersed in 30 ml of Dulbecco's phosphate buffer solution (D-PBS) containing 25 mM glucose and the same concentration of different bioflavonoids, viz., diosmin, quercetin, chrysin, Methyl hesperidin and gossypin, was introduced into the mucosal side. Transport of nitrendipine from serosal to mucosal surfaces across the intestine was determined by collecting samples from the mucosal medium periodically at different intervals: 0, 10, 20, 30, 60, 90 and 120 minutes. The samples were analyzed by HPLC. Diosmin and quercetin decreased the transport rate of nitrendipine to nearly the same extent in all regions. Chrysin and gossypin decreased the transport rate of nitrendipine to a greater extent in the ileum than in the duodenum and jejunum. Methyl hesperidin caused inhibition of nitrendipine transport in the ileum and jejunum, but not in the duodenum.
CONCLUSIONS:
All bioflavonoids, i.e., quercetin, diosmin, Methyl hesperidin, gossypin and chrysin, decreased the transport of nitrendipine, a P-gp substrate in the rat intestine. The highest expression of P-gp was found in the ileum followed by the jejunum and duodenum.
Food Chem Toxicol. 1990 Sep;28(9):613-8.
Carcinogenicity study of methyl hesperidin in B6C3F1 mice.
METHODS AND RESULTS:
A long-term carcinogenicity study of Methyl hesperidin, a compound of the vitamin P group, was carried out in B6C3F1 mice receiving dietary concentrations of 0, 1.25 or 5%. Administration was continued for 96 wk and then the mice were maintained on basal diet for an additional 8 wk. Growth retardation during the experiment with final changes in organ weights were observed in females given the 1.25% dose of Methyl hesperidin and in both sexes receiving the 5.0% treatment. However, no biologically significant effects were evident with respect to mortality or clinical signs. Furthermore, treatment with Methyl hesperidin did not result in any changes in haematology, clinical chemistry and urinalysis data. On histological examination, no significant alteration of non-neoplastic and neoplastic lesion incidence was observed in treated mice.
CONCLUSIONS:
The results thus demonstrated that Methyl hesperidin lacked any carcinogenicity for B6C3F1 mice in the 96-wk feeding regimen used in this study.
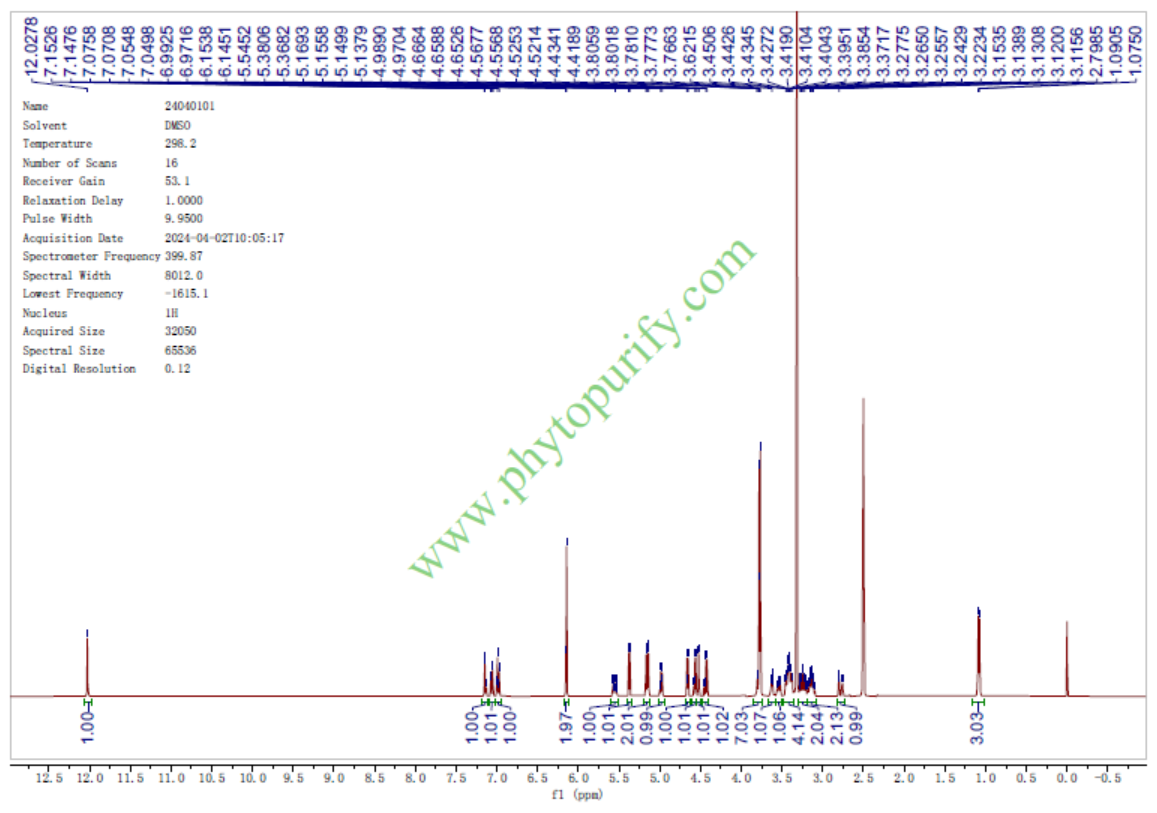
HNMR of Methyl Hesperidin