
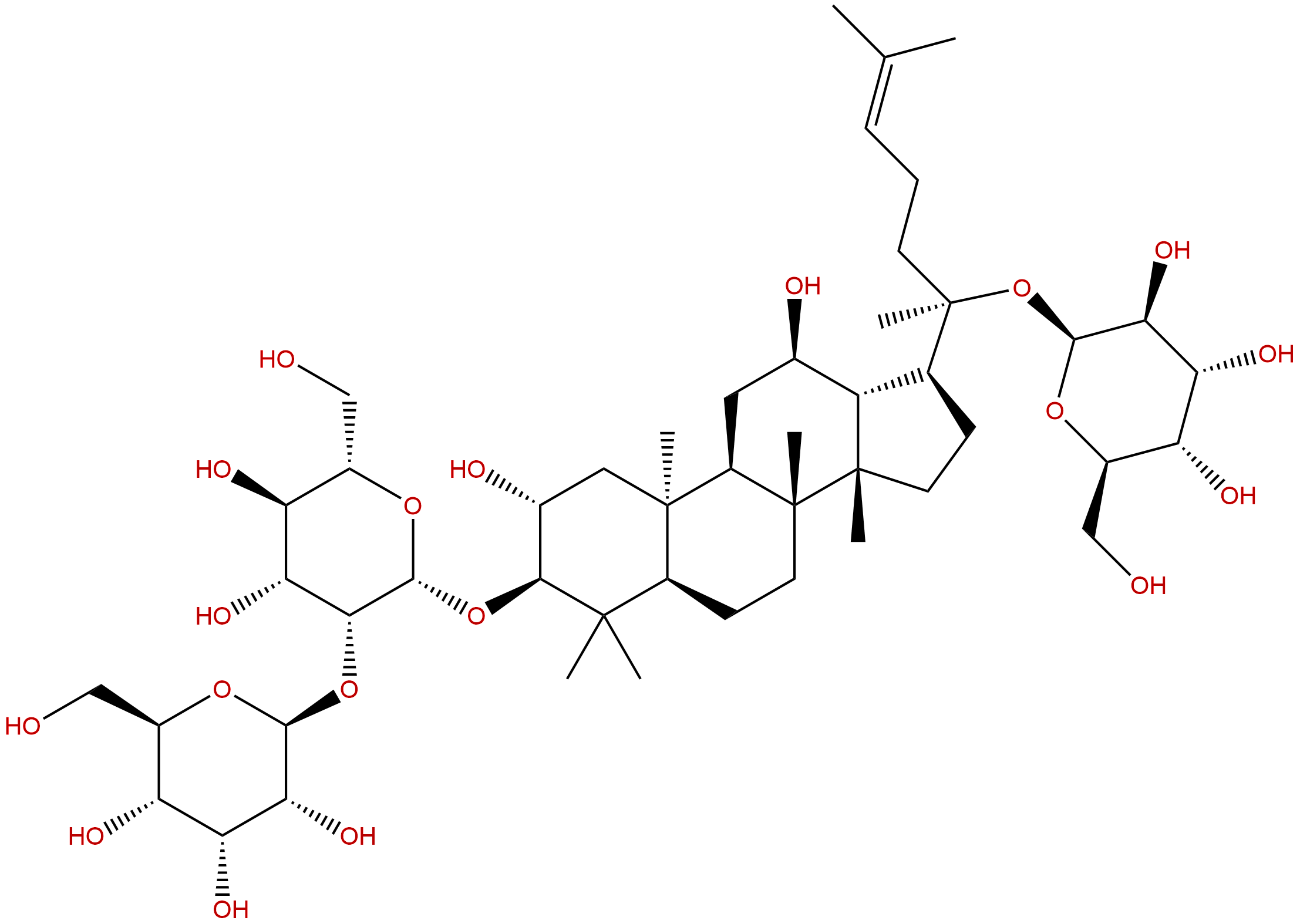
Gypenoside XLVICAS No.:94705-70-1
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1791 |
| Formula: | C48H82O19 |
| Mol Weight: | 963.165 |
Product name: Gypenoside XLVI
Synonym name:
Catalogue No.: BP1791
Cas No.: 94705-70-1
Formula: C48H82O19
Mol Weight: 963.165
Botanical Source: Gynostemma pentaphyllum
Physical Description:
Type of Compound: Triterpenoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
The product could be supplied from milligrams to grams. Inquire for bulk scale.
We provide solution to improve the water-solubility of compounds, thereby facilitating the variety of activity tests and clinic uses.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
Gypenoside XLVI shows strong cytotoxic activity against A549 cells, with the IC50 values of 52.63±8.31 ug/ml.
References:
Faseb J., 2014, 28(1).
Cytotoxic activity of gypenosides and gynogenin from Gynostemma pentaphyllum against A549 cells
Gynostemma pentaphyllum (Thunb.) Makino is one of the well-known traditional Chinese herbal medicines, which has been demonstrated to be effective against chronic diseases, such as lung cancer. In the present study, seven dammarane-type compounds were isolated from the raw, heat processed and alkaline hydrolyzed G. Pentaphyllum and compared for their cytotoxic activities against non-small cell lung carcinoma A549 cells using MTT cytotoxicity assay.
METHODS AND RESULTS:
By means of chemical and spectroscopic methods, their structures were established as gypenoside LVI, Gypenoside XLVI, gypenoside L, gypenoside LI, damulin A, damulin B and 20S-dammar-24-en-2α, 3β, 12β, 20-tetrol. Gypenoside LVI, Gypenoside XLVI, gypenoside L, gypenoside LI, damulin A, damulin B and 20S-dammar-24-en-2α,3β,12β,20-tetrol all showed strong cytotoxic activity against A549 cells, with the IC50 values of 105.89±2.48, 52.63±8.31, 34.94±4.23, 50.96±9.55, 26.98±0.51, 4.56±0.58, 12.54±0.53 μg/ml respectively, whereas the IC50 value of 20S-Rg3 (positive control) was 55.36±3.20 μg/ml. Comparing their structures with cytotoxic activity, these compounds might possess some kind of structure-activity relationship.
CONCLUSIONS:
Based on the structure and cytotoxic activity relationships of these seven constituents, the OH group in C-2, the double bond in C20(21) and C20(22) positions, the connected sugar number and the configuration in C-20 were important for cytotoxic activity against A549 cells.
HPLC of Gypenoside XLVI
