
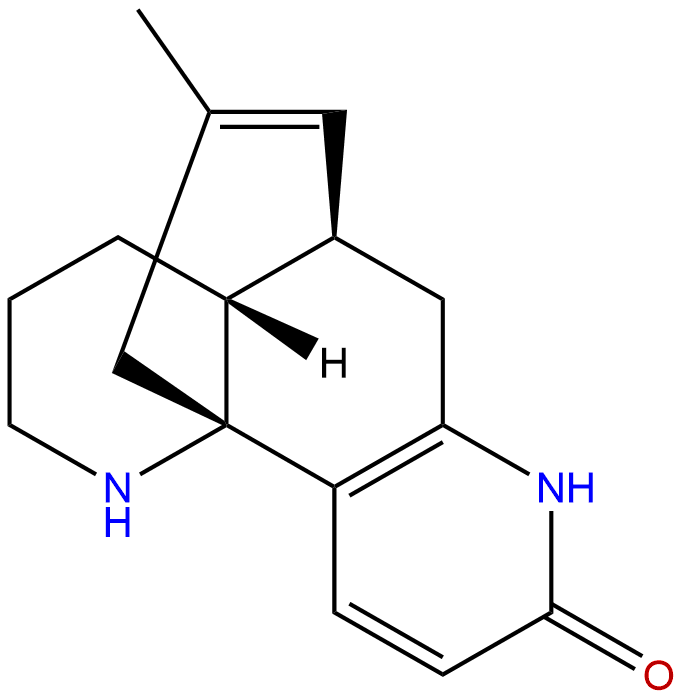
Huperzine BCAS No.:103548-82-9
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0741 |
| Formula: | C16H20N2O |
| Mol Weight: | 256.349 |
Synonym name: Fordimine
Catalogue No.: BP0741
Cas No.: 103548-82-9
Formula: C16H20N2O
Mol Weight: 256.349
Botanical Source: Alkaloid from Lycopodium serratum, Huperzia serrata and Phlegmariurus fordii (preferred genus name Huperzia)
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Description:
Huperzine B is a efficient inhibitor of human brain AChE, it can enhance ognitive and protect neuro, may be potentially new drug candidates for Alzheimer's disease therapy.
References:
Acta Pharmacol Sin. 2009 Aug;30(8):1195-203.
Novel 16-substituted bifunctional derivatives of huperzine B: multifunctional cholinesterase inhibitors.
To design novel bifunctional derivatives of Huperzine B (HupB) based on the concept of dual binding site of acetylcholinesterase (AChE) and evaluate their pharmacological activities for seeking new drug candidates against Alzheimer's disease (AD).
METHODS AND RESULTS:
Novel 16-substituted bifunctional derivatives of Huperzine B were synthesized through chemical reactions. The inhibitory activities of the derivatives toward AChE and butyrylcholinesterase (BuChE) were determined in vitro by modified Ellman's method. Cell viability was quantified by the reduction of MTT. A new preparative method was developed for the generation of 16-substituted derivatives of Huperzine B, and pharmacological trials indicated that the derivatives were multifunctional cholinesterase inhibitors targeting both AChE and BuChE. Among the derivatives tested, 9c, 9e, 9f, and 9i were 480 to 1360 times more potent as AChE inhibitors and 370 to 1560 times more potent as BuChE inhibitors than the parent Huperzine B. Further preliminary pharmacological trials of derivatives 9c and 9i were performed, including examining the mechanism of AChE inhibition, the substrate kinetics of the enzyme inhibition, and protection against hydrogen peroxide (H2O2)-induced cytotoxicity in PC12 cells.
CONCLUSIONS:
Preliminary pharmacological evaluation indicated that 16-substituted derivatives of Huperzine B, particularly 9c and 9i, would be potentially valuable new drug candidates for AD therapy, and further exploration is needed to evaluate their pharmacological and clinical efficacies.
Bioorg Med Chem. 2007 Feb 1;15(3):1394-408.
Study on dual-site inhibitors of acetylcholinesterase: Highly potent derivatives of bis- and bifunctional huperzine B.
Natural (-)-Huperzine B (HupB), isolated from Chinese medicinal herb, displayed moderate inhibitory activity of acetylcholinesterase (AChE).
METHODS AND RESULTS:
Based on the active dual-site of AChE, a series of novel derivatives of bis- and bifunctional HupB were designed and synthesized. The AChE inhibition potency of most derivatives of HupB was enhanced about 2-3 orders of magnitude as compared with the parental HupB. Among bis-HupB derivatives, 12h exhibited the most potent in the AChE inhibition and has been evaluated for its pharmacological actions in vivo on ChE inhibition, cognitive enhancement, and neuroprotection.
CONCLUSIONS:
The docking study on the bis-HupB derivatives 12 series with TcAChE has demonstrated that the ligands bound to the dual-site of the enzyme in different level.