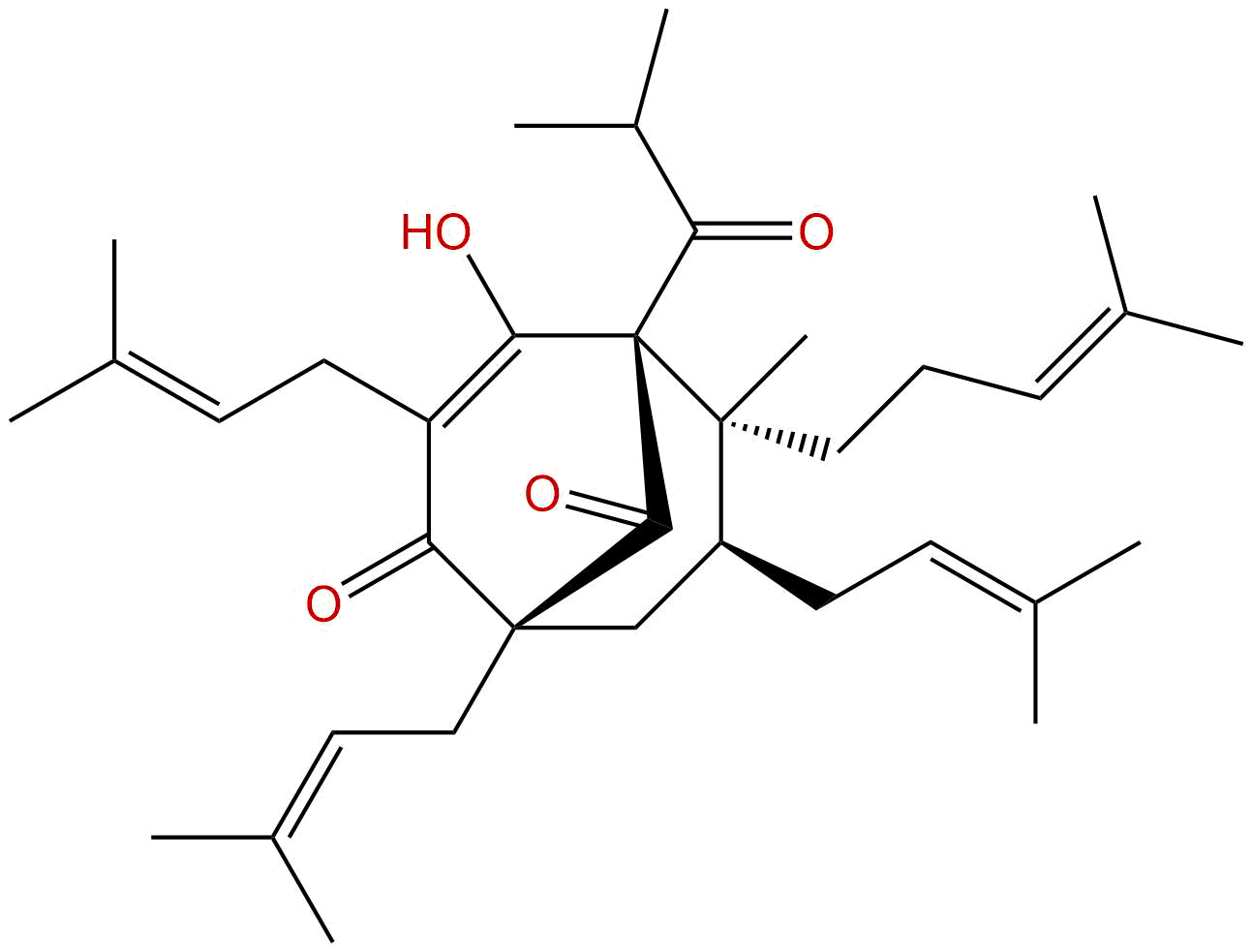
HyperforinCAS No.:11079-53-1
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0751 |
| Formula: | C35H52O4 |
| Mol Weight: | 536.797 |
Synonym name:
Catalogue No.: BP0751
Cas No.: 11079-53-1
Formula: C35H52O4
Mol Weight: 536.797
Botanical Source: Hypericum perforatum (St John's Wort)
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Hyperforin is a polyprenylated acylphloroglucinol derivative from Hypericum perforatum (St. John's wort), it is a member of the Polycyclic polyprenylated acylphloroglucinol family, also known as the PPAP family. Hyperforin is unstable in the presence of light and oxygen. Frequent oxidized forms contain a C3 to C9 hemiketal/heterocyclic bridge or will form furan/pyran derivatives.
Hyperforin exhibits antidepressant activity by a novel mechanism of action, antibiotic activity against gram-positive bacteria, and antitumoral activity in vivo. However, it also produces drug-drug interactions by activation of the pregnan X receptor. No total synthesis has been described. Some natural and semisynthetic analogues are available to study structure-activity relationships. Enzymatically, the skeleton of hyperforin is formed by isobutyrophenone synthase from isobutyryl-CoA and three molecules of malonyl-CoA. The first prenylation step is catalyzed by a soluble and ion-dependent dimethylallyltransferase. Hyperforin mainly accumulates in pistils and fruits where it probably serves as defensive compound.
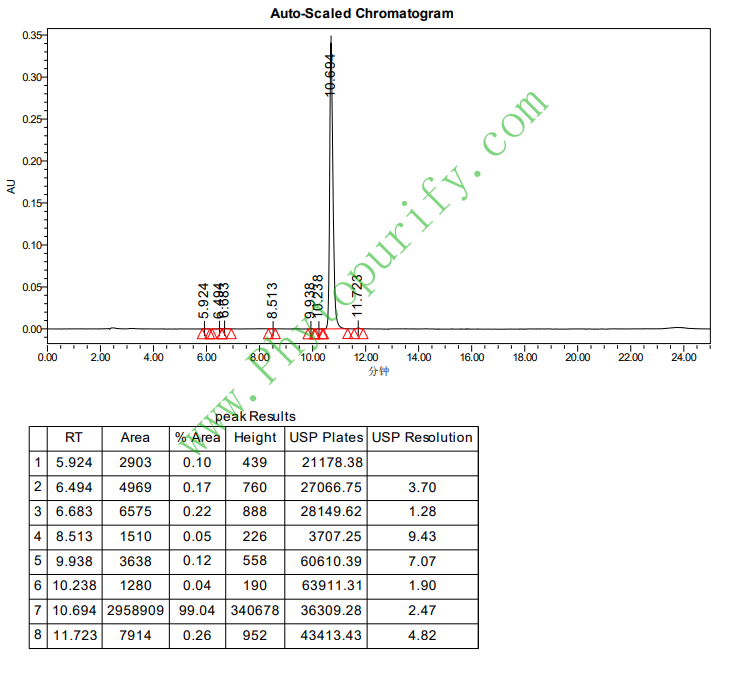
HPLC of Hyperforin