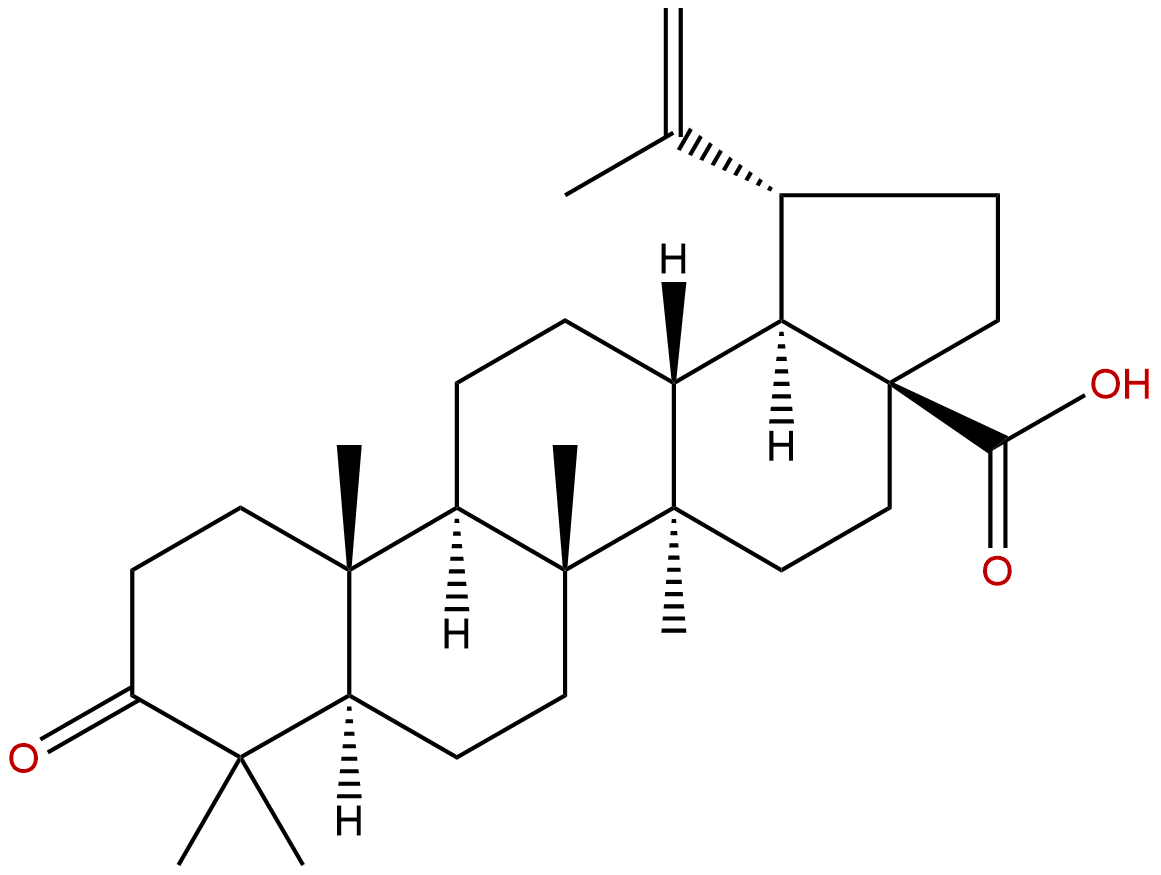
Liquidambaric acidCAS No.:4481-62-3
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0871 |
| Formula: | C30H46O3 |
| Mol Weight: | 454.695 |
Product name: Liquidambaric acid
Synonym name: Betulonic acid
Catalogue No.: BP0871
Cas No.: 4481-62-3
Formula: C30H46O3
Mol Weight: 454.695
Botanical Source: Liquidambar formosana Hance
Physical Description:
Type of Compound: Triterpenoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Betulonic acid has anti-cancer , anti-HIV,hepatoprotective and anti-inflammatory activities, it has antiviral activity against herpes simplex virus, it also suppresses ECHO 6 virus reproduction. Betulonic acid derivatives have a promising cytostatic activity in vitro and could be used as potential leads for the development of new type of anti-cancer agents.
References:
Bioorg Med Chem. 2014 Jul 1;22(13):3292-300.
Synthesis of triterpenoid triazine derivatives from allobetulone and betulonic acid with biological activities.
The synthetic transformation and modification of natural products with the aim to improve the biological properties is an area of current interest. The triterpenoids betulin and betulinic acid are very abundant in nature and now are commercially available.
METHODS AND RESULTS:
In our study, starting from betulin and betulinic acid, we obtained allobetulone and Betulonic acid in a few synthetic steps. The ketone function at the A-ring was used as the starting point for the synthesis of a series of 1,2,4-triazine-fused triterpenoids. The alkylation and Liebeskind-Srogl coupling were used for further substitution of 1,2,4-triazines, and the intramolecular hetero Diels-Alder reaction leads to interesting fused thienopyridine derivatives. All new compounds were tested for their cytostatic activities against murine leukemia L1210, human cervix carcinoma HeLa and human lymphoblast CEM tumor cells.
CONCLUSIONS:
The results show that some triterpenoid triazine Betulonic acid derivatives have a promising cytostatic activity in vitro and could be used as potential leads for the development of new type of anti-cancer agents. Several compounds were also endowed with anti-HCMV activity in the low micromolar range.
Fitoterapia. 2003 Jul;74(5):489-92.
Antiviral activity of betulin, betulinic and betulonic acids against some enveloped and non-enveloped viruses.
METHODS AND RESULTS:
Antiviral properties of betulin, betulinic and Betulonic acids were investigated in cell cultures infected with herpes simplex type I, influenza FPV/Rostock and ECHO 6 viruses.
CONCLUSIONS:
All studied triterpenes were active against herpes simplex virus. Betulin and especially betulinic acid also suppressed ECHO 6 virus reproduction.
Bioorg Med Chem. 2009 Jul 15;17(14):5164-9.Efficient synthesis of the first betulonic acid-acetylene hybrids and their hepatoprotective and anti-inflammatory activity.
The Sonogashira reaction can be applied for the preparation of acetylenic derivatives of Betulonic acid where the triterpenoid moiety can serve as either the halo- or the acetylenic component. This reaction opened access to the first derivatives of Betulonic acid containing either the arylethynyl (C[triple bond]C-Ar(Het) or the ethynyl (C[triple bond]CH) moieties. From the fundamental perspective, this work illustrates the possibility of selective Pd-catalyzed cross-coupling at terminal acetylenes in the presence of a terminal alkene.
METHODS AND RESULTS:
Hepatoprotective and anti-inflammatory properties of selected acetylenic derivatives of Betulonic acid were investigated using the CCl4-induced hepatitis and carrageenan-induced edema models, respectively.
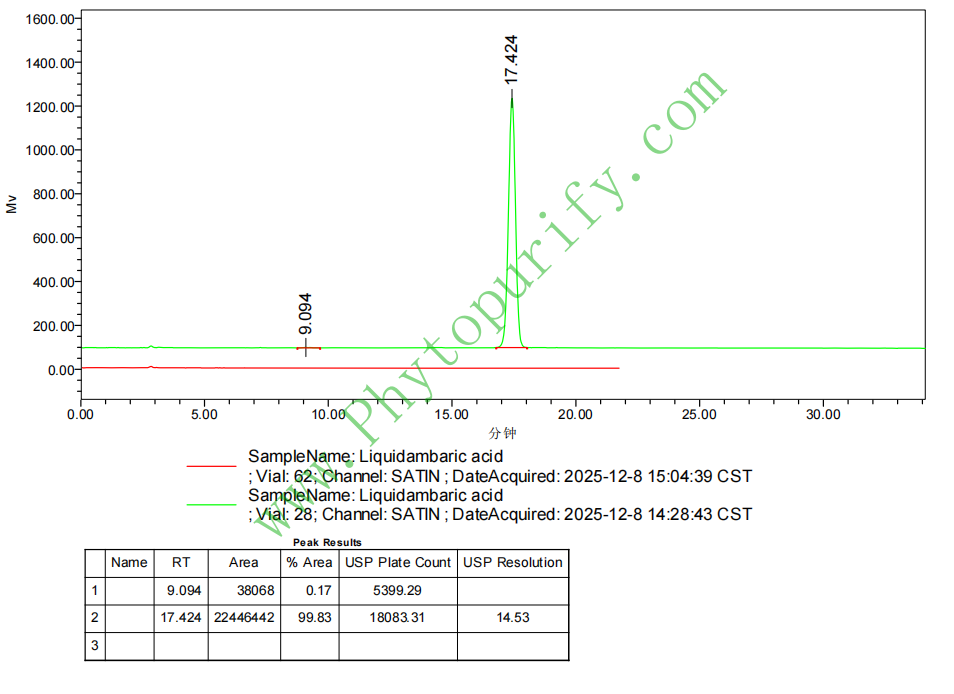
HPLC of Liquidambaric acid