
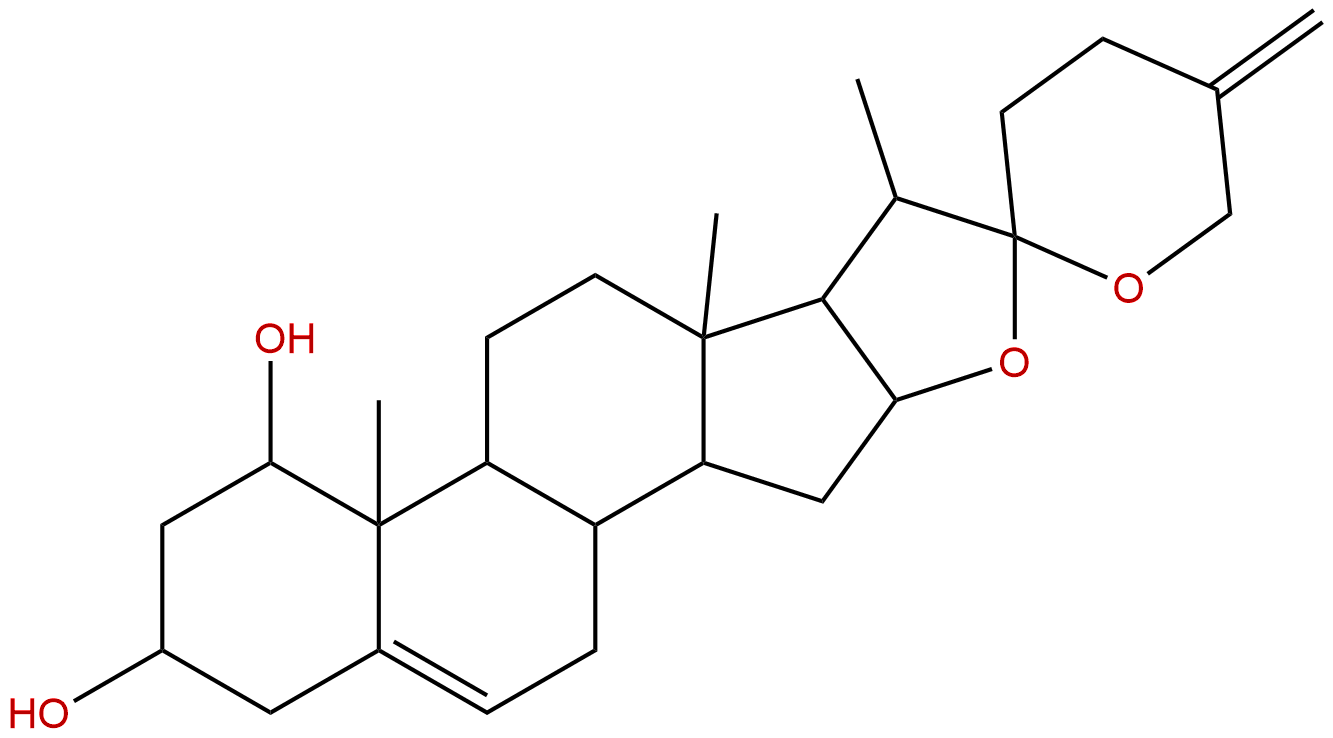
NeoruscogeninCAS No.:17676-33-4
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0994 |
| Formula: | C27H40O4 |
| Mol Weight: | 428.613 |
Synonym name:
Catalogue No.: BP0994
Cas No.: 17676-33-4
Formula: C27H40O4
Mol Weight: 428.613
Botanical Source: Ruscus aculeatus, Ruscus hypoglossum and Sansevieria trifasciata
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Description:
Neoruscogenin represents a universal pharmacological tool for RORα research due to its specific selectivity profile versus other nuclear receptors.
References:
J Biomol Screen. 2014 Mar;19(3):399-406.
The identification of naturally occurring neoruscogenin as a bioavailable, potent, and high-affinity agonist of the nuclear receptor RORα (NR1F1).
Plants represent a tremendous structural diversity of natural compounds that bind to many different human disease targets and are potentially useful as starting points for medicinal chemistry programs. This resource is, however, still underexploited due to technical difficulties with the identification of minute quantities of active ingredients in complex mixtures of structurally diverse compounds upon raw phytomass extraction.
METHODS AND RESULTS:
In this work, we describe the successful identification of a novel class of potent RAR-related orphan receptor alpha (RORα or nuclear receptor NR1F1) agonists from a library of 12,000 plant extract fractions by using an optimized, robust high-throughput cell-free screening method, as well as an innovative hit compound identification procedure through further extract deconvolution and subsequent structural elucidation of the active natural compound(s). In particular, we demonstrate that Neoruscogenin, a member of the steroidal sapogenin family, is a potent and high-affinity RORα agonist, as shown by its activity in RORα reporter assays and from its effect on RORα target gene expression in vitro and in vivo.
CONCLUSIONS:
Neoruscogenin represents a universal pharmacological tool for RORα research due to its specific selectivity profile versus other nuclear receptors, its excellent microsomal stability, good bioavailability, and significant peripheral exposure in mouse.
Phytochemistry. 2013 Jun;90:106-13.
Spirostanol saponins and esculin from Rusci rhizoma reduce the thrombin-induced hyperpermeability of endothelial cells.
Rusci rhizoma extracts are traditionally used against chronic venous disorders (CVD).
METHODS AND RESULTS:
To determine the effect of its secondary plant metabolites on the endothelium, phenolic compounds and saponins from Butcher's broom were isolated from a methanolic extract, and their activity on the thrombin-induced hyperpermeability of human microvascular endothelial cells (HMEC-1) was investigated in vitro. In addition to the six known spirostanol saponins deglucoruscin (5), 22-O-methyl-deglucoruscoside (6), deglucoruscoside (7), ruscin (8), ruscogenin-1-O-(α-l-rhamnopyranosyl-(1→2)-β-d-galactopyranoside (9) and 1-O-sulpho-ruscogenin (10), three new spirostanol derivatives were isolated and identified: 3'-O-acetyl-4'-O-sulphodeglucoruscin (1), 4'-O-(2-hydroxy-3-methylpentanoyl)-deglucoruscin (2) and 4'-O-acetyl-deglucoruscin (3). Furthermore, the coumarin esculin (4), which is also prominently present in other medicinal plants used in the treatment of CVD, was isolated for the first time from Rusci rhizoma. Five of the isolated steroid derivatives (2, 5, 8, 9 and 10) and esculin (4) were tested for their ability to reduce the thrombin-induced hyperpermeability of endothelial cells in vitro, and the results were compared to those of the aglycone Neoruscogenin (11). The latter compound showed a slight but concentration-dependent reduction in hyperpermeability to 71.8% at 100μM. The highest activities were observed for the spirostanol saponins 5 and 8 and for esculin (4) at 10μM, and these compounds resulted in a reduction of the thrombin-induced hyperpermeability to 41.9%, 42.6% and 53.3%, respectively. For 2, 5 and 8, the highest concentration tested (100μM) resulted in a drastic increase of the thrombin effect. The effect of esculin observed at a concentration of 10μM was diminished at 100μM.
CONCLUSIONS:
These in vitro data provide insight into the pharmacological mechanism by which the genuine spirostanol saponins and esculin can contribute to the efficacy of Butcher's broom against chronic venous disorders.