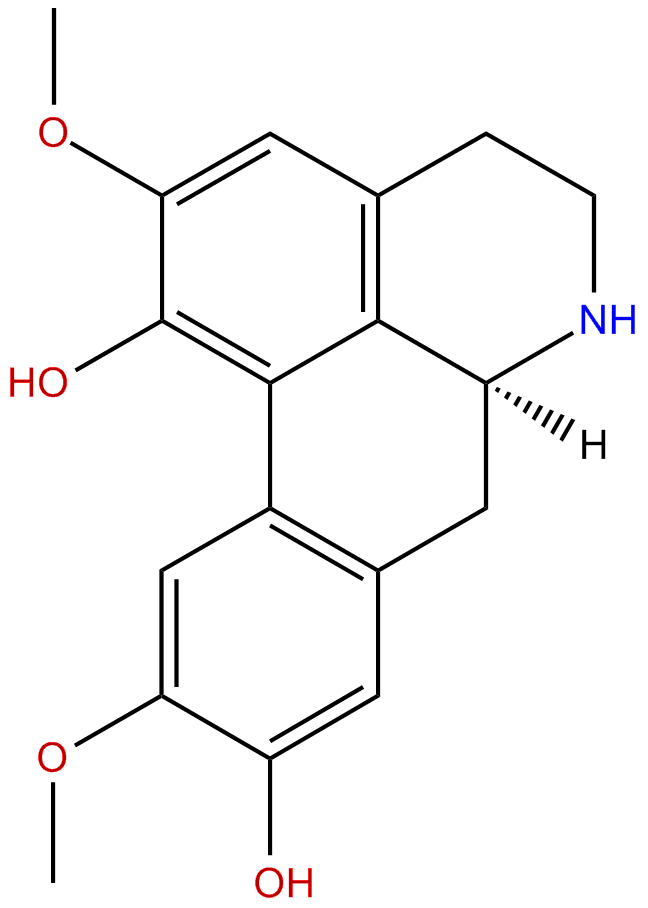
NorisoboldineCAS No.:23599-69-1
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1007 |
| Formula: | C18H19NO4 |
| Mol Weight: | 313.353 |
Product name: Norisoboldine
Synonym name: Laurelliptine
Catalogue No.: BP1007
Cas No.: 23599-69-1
Formula: C18H19NO4
Mol Weight: 313.353
Botanical Source: Alkaloid from Beilschmiedia elliptica, Beilschmiedia podagrica, Cassytha pubescens, Monodora tenuifolia, Litsea xylanica and some other Litsea spp., Zizyphus jujuba, Nectandra rigida and (possibly) Monanthotaxis cauliflora (Lauraceae, Annonaceae, Rhamnac
Physical Description: Powder
Type of Compound: Alkaloids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams, up to kilograms
Inquire for bulk scale.
Descriptions:
Norisoboldine is the main isoquinoline alkaloid occurring in Radix Linderae, the dry roots of Lindera aggregata (Lauraceae family),it has been previously implicated to be able to ameliorate the synovial inflammation and abnormal immune conditions in collagen-induced arthritis of mice; it inhibits the macrophage activation and the resultant production of pro-inflammatory cytokines via down-regulating the activation of MAPKs signaling pathways rather than NF-κB.[1]
Norisoboldine can suppress osteoclast differentiation through preventing the accumulation of TRAF6-TAK1 complexes and activation of MAPKs/NF-κB/c-Fos/NFATc1 pathway; it alao inhibits the production of interleukin-6 in fibroblast-like synoviocytes from adjuvant arthritis rats through PKC/MAPK/NF-κB-p65/CREB pathway.[2,3]
Norisoboldine can significantly alleviate the severity of collagen II -induced arthritis (CIA) , based on the reduced clinical scores and elevated the lowered body weights of model mice, it also significantly suppressed the enhanced T cell responses in vivo, suggests that norisoboldine might be a potential therapeutic agent for rheumatoid arthritis, and it functions through protecting joint destruction as well as regulating the abnormal immune responses.[4]
Norisoboldine can alleviate joint destruction in AIA rats by reducing RANKL, IL-6, PGE2, and MMP-13 expression via the p38/ERK/AKT/AP-1 pathway.[5]
Norisoboldine attenuates inflammatory pain and decreases forskolin-evoked cyclic adenosine monophosphate levels in mouse spinal cord neuronal cultures through the adenosine A1 receptor.[6]
Norisoboldine inhibit VEGF-induced endothelial cell migration via a cAMP-PKA-NF-κB/Notch1 signaling pathway.[7]
References:
[1] Luo Y, Liu M, Dai Y, et al. Inflammation, 2010, 33(6):389-97.
[2] León M, Martín P, Bravo D, et al. Plos One, 2013, 8(3):e59171-e59171.
[3] Wei Z F, Wang FY, Song J, et al. J Cell Biochem, 2012, 113(8):2785-95.
[4] Luo Y, Liu M, Xia Y, et al. Phytomedicine International Journal of Phytotherapy & Phytopharmacology, 2010, 17(10):726-31.
[5] Wei Z F, Jiao X L, Wang T, et al. Acta Pharmacol Sin, 2013, 34(3):403-13.
[6] Gao X, Lu Q, Chou G, et al. Eur J Pain, 2014, 18(7):939-48.
[7] Lu Q, Tong B, Luo Y, et al. Plos One, 2013, 8(12):e81220.
[8] Chen J, Guixin C, Yang L, et al. China Journal of Chinese Materia Medica, 2009, 34(21):2774-6.
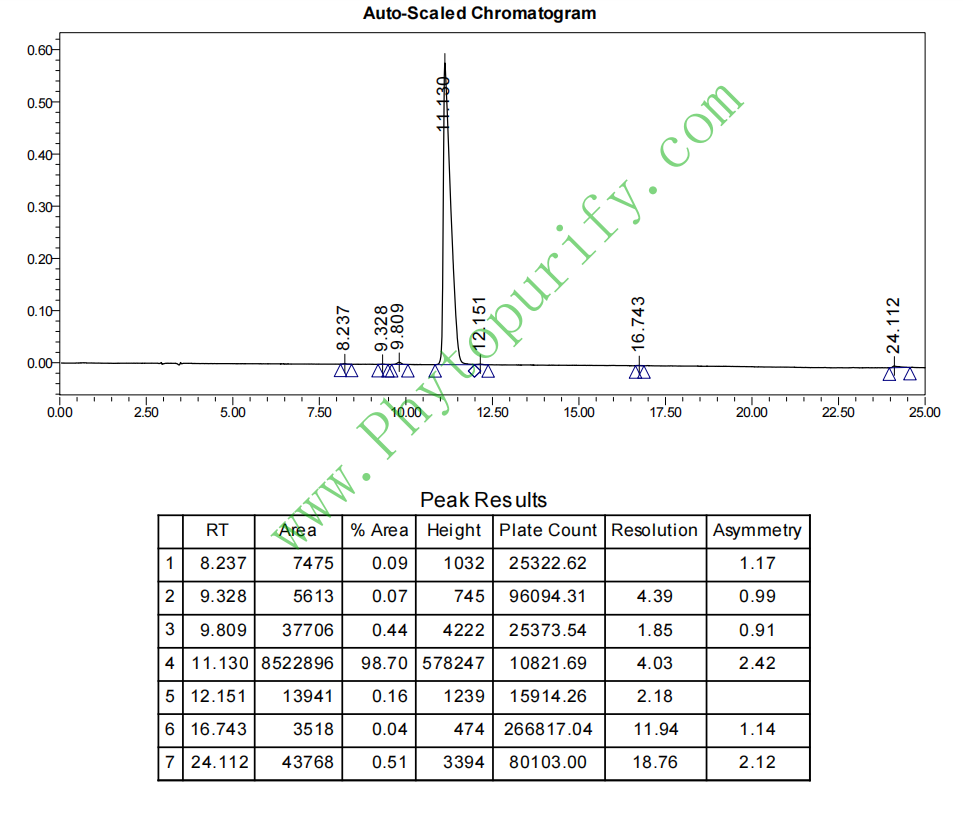
HPLC of Norisoboldine