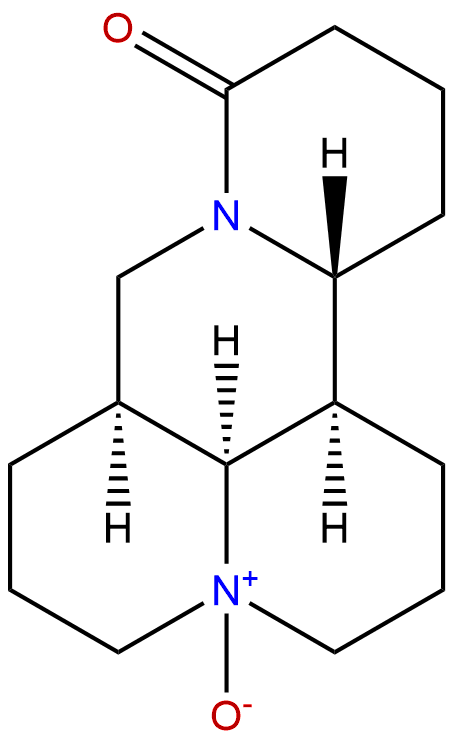
OxymatrineCAS No.:16837-52-8
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1049 |
| Formula: | C15H24N2O2 |
| Mol Weight: | 264.369 |
Product name: Oxymatrine
Synonym name: Ammothamnine; Pachycarpidine
Catalogue No.: BP1049
Cas No.: 16837-52-8
Formula: C15H24N2O2
Mol Weight: 264.369
Botanical Source: Alkaloid from Sophora flavescens, Sophora macrocarpa, Sophora alopecuroides, Ammothamnus lehmannii (preferred genus name Sophora), Euchresta horsfeldii and other plants (Leguminosae)
Physical Description: Powder
Type of Compound: Alkaloids
Purity: 95%~99%
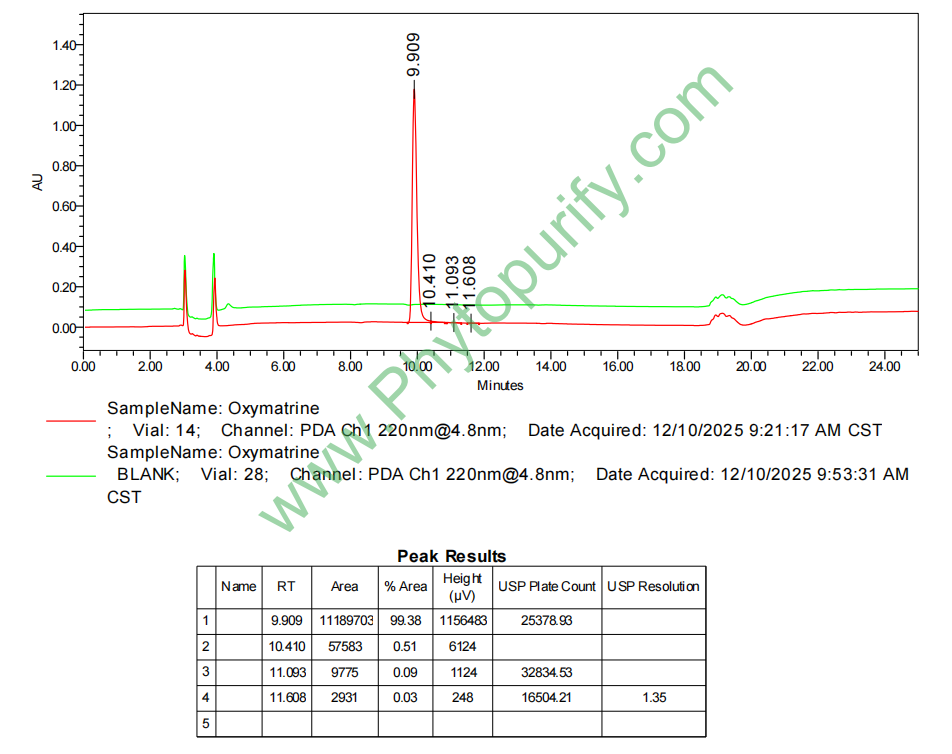
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams, up to kilograms
Inquire for bulk scale.
Descriptions:
Oxymatrine, one of the major components of Sophora flavescens ait, has exhibited anti-hepatitis virus infection, anti-hepatic fibrosis, anti-inflammation, anti-anaphylaxis and other immune-regulation, it induces human pancreatic cancer PANC-1 cells apoptosis via regulating expression of Bcl-2 and IAP families, and releasing of cytochrome C.[1]
Oxymatrine is proven to protect ischemic and reperfusion injury in liver, intestine and heart, this effect is via anti-inflammation and anti-apoptosis, it has protective effect applies to ischemic injury in brain to reduce infarct volume induced by pMCAO, this effect may be through the decreasing of NF-kappaB expression.[2]
Oxymatrine has anti-inflammatory activity, it can reduce the serum levels of TNF-α, IL-6, and the expression of NF-κB and ICAM-1 in colonic mucosa in dextran sulfate sodium (DSS)--induced colitis of rats, indicates that oxymatrine may ameliorate the colonic inflammation and thus alleviate diarrhea and bloody stool.[3]
Oxymatrine has a beneficial effect on acute lung injury induced by oleic acid in mice and may inhibit the production of proinflammatory cytokine, TNF-alpha, by means of the inhibition of p38 MAPK.[4]
Oxymatrine has inhibition of hepatitis B virus in vivo, it (200 mg/kg/d,20day )can reduce thecontents of HBsAg and HBcAg in transgenic miceliver, and longer treatment time and larger dosage do not yield better effects.[5]
Oxymatrine can attenuate diabetes-associated cognitive deficits in rats, which is associated with oxidative stress, inflammation and apoptotic cascades.[6]
Oxymatrine can induce cell cycle arrest and apoptosis, which makes it a potentially useful agent for targeting cancer cells; it causes a dose-dependent reduction in the proliferation of MCF-7 cells and a decrease in SP cells, the growth inhibitory effects of oxymatrine treatment on MCF-7 cells may be due to the inhibition of SP and Wnt/0205-catenin signaling pathway. [7]
Oxymatrine and astragalus polysaccharide can synergistically improve the immune efficacy of Newcastle disease vaccine in chicken, because of astragalus polysaccharide and oxymatrine possesses synergistical immunoenhancement.[8]
References:
[1] Qi L, Xiao X, Wei X, et al. J Exp Clin Cancer Res, 2011, 30(1):66-66.
[2] Zhang X J, Yang C H, Fan H G. Brain Res, 2009, 1268:174-80.
[3] Zheng P, Niu F L, Liu W Z, et al. World J Gastroenterol, 2005, 11(31):4912-5.
[4] Lao L, Rao S Y, Gong Z N, et al. J Ethnopharmacol, 2005, 98(1-2):177-83.
[5] Xiao, Song, Chen, et al. World J Gastroenterol, 2001, 7(1):49-52.
[6] Suo-bin, WANG, Jian-ping. Acta Pharmacol Sin, 2014, 35(3):331-8.
[7] Zhang Y, Piao B, Zhang Y, et al. Med Oncol, 2011, 28(1):99-107.
[8] Chen Y, Wang D, Hu Y, et al. Int J Biol Macromol, 2010, 46(4):425-8.
[9] Liu J, Yuan L M, Li-Qin H E, et al. Chemical World, 2009, 50(7):410-1.
HPLC of Oxymatrine