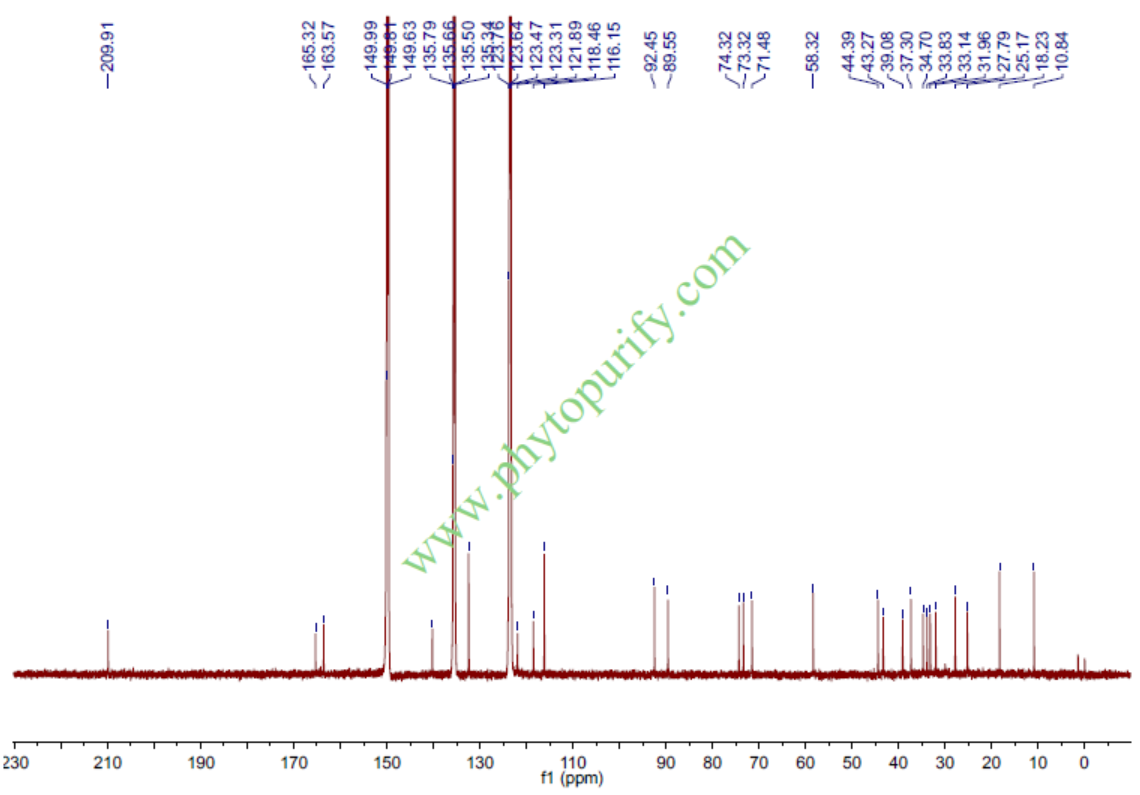
CynanchageninCAS No.:84745-94-8
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1185 |
| Formula: | C28H36O8 |
| Mol Weight: | 500.588 |
Synonym name: Qingyangshengenin
Catalogue No.: BP1185
Cas No.: 84745-94-8
Formula: C28H36O8
Mol Weight: 500.588
Botanical Source: Cynanchum otophyllum, Cynanchum wallichii and Marsdenia incisa
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Description:
Qingyangshengenin is a a glycoside from the roots of Cynanchum otophyllum.
References:
J Asian Nat Prod Res. 2013;15(1):71-7.
Chemical constituents of Arisaema franchetianum tubers.
A novel pyrrolidine alkaloid, (2R*,3S*,5S*)-N,2-dimethyl-3-hydroxy-5-(10-phenyldecyl)pyrrolidine (1), and 17 known compounds were isolated from Arisaema franchetianum Engl. (Araceae) tubers. The 17 compounds were bergenin (2), emodin (3), caffeic acid (4), nobiletin (5), 3-O-β-d-galactopyranosyl-hederagenin 28-O-β-d-xylopyranosyl(1 → 6)-β-d-galactopyranosyl ester (6), coniferin (7), Qingyangshengenin (8), methylconiferin (9), syringaresinol 4'-O-β-d-glucopyranoside (10), gagaminine (11), perlolyrine (12), (S)-1-(1'-hydroxyethyl)-β-carboline (13), 1-(β-carboline-1-yl)-3,4,5-trihydroxy-1-pentanone (14), 1-methoxycarbonyl-β-carboline (15), indolo[2,3-α]carbazole (16), 4-hydroxycinnamic acid methyl ester (17), and methyl 4-[2-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-1-(hydroxymethyl)ethyl] ferulate (18).
METHODS AND RESULTS:
The inhibitory activities of compound 1 and its N-methyl derivative (1a) against porcine respiratory and reproductive syndrome virus (PRRSV), human leukemic K562 cells, and human breast cancer MCF-7 cells were evaluated. Compounds 1 [50% inhibited concentration (IC(50)) = 12.5 ± 0.6 μM] and 1a (IC(50) = 15.7 ± 0.9 μM) were cytotoxic against K562 cells. Compound 1a also had a weak effect on PRRSV with an IC(50) value of 31.9 ± 6.0 μM [selectivity index (SI) = 18.7].
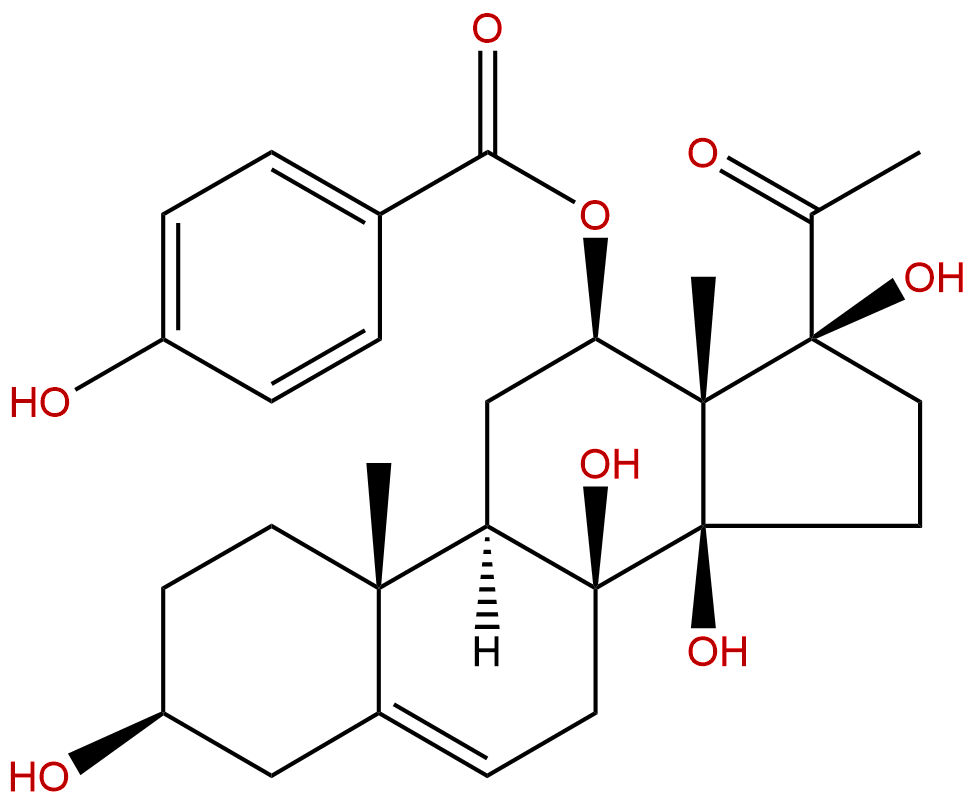
HPLC of Cynanchagenin
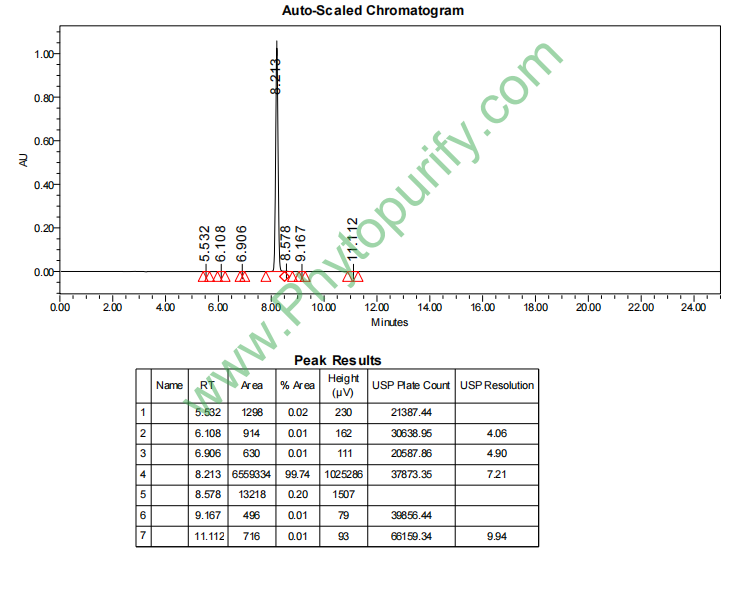
NMR of Cynanchagenin