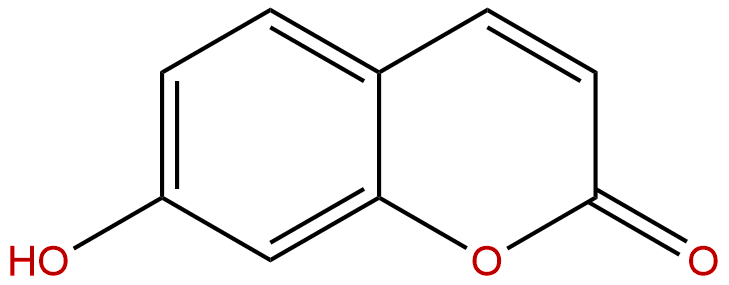
7-HydroxycoumarinCAS No.:93-35-6
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0101 |
| Formula: | C9H6O3 |
| Mol Weight: | 162.144 |
Product name: 7-Hydroxycoumarin
Synonym name: Umbelliferone; Dichrin A; Hydrangin; Skimmetin
Catalogue No.: BP0101
Cas No.: 93-35-6
Formula: C9H6O3
Mol Weight: 162.144
Botanical Source: Occurs widely in plants including Angelica, Artemisia, Ferula and Ruta spp., Hieracium pilosella, Coronilla varia, Picea abies, Peddiea fischeri, Lepidophyllum cupressiforme, Cymbopogon parkeri, Wikstroemia elliptica, Euphorbia humifusa,
Physical Description: Powder
Type of Compound: Coumarins
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams, up to kilograms
Inquire for bulk scale.
Descriptions:
Umbelliferone (UMB), a natural antioxidant, is benzopyrone in nature, and it is present in the fruits of golden apple and bitter orange, UMB has a protective effect on membrane fatty acid composition of liver and kidney as supported by antioxidant and antihyperlipidemic effects of UMB reported earlier as evidenced by improved histopathological changes, hepatic and nephritic markers, indicating recovery from the risk of diabetic complications; UMB has antihyperglycemic effect, UMB at 30 mg/kg of body weight possesses a promising antihyperglycemic effect that is comparable with glibenclamide.[1,2]
Umbelliferone analogues and their potential to be antimutagenic/anticarcinogenic against mutations induced by benzo(a)pyrene, a potent mutagen/carcinogen, and hydrogen peroxide, and for their ability to function as free radical scavengers. [3]
Umbelliferone has antioxidative effect, the antioxidative effect is dose-dependent up to 100 ug/ml and then levelled off with no further increase in activity. [4]
Umbelliferone, ferulic acid and eugenol are competitive and limonene is a competitive–non-competitive inhibitor of alkaline phosphatase, ferulic acid and umbelliferone are competitive, whereas eugenol and limonene are competitive–non-competitive and uncompetitive inhibitors of acetylcholinesterase, respectively. [5]
Umbelliferone derivatives is a novel class of inhibitors for steroid 5α-reductase.[6]
Umbelliferone attenuates the alteration characteristics of allergic airway inflammation,
the mechanisms of action of this molecule may contribute for the development of new drugs for the treatment of asthma.[7]
Umbelliferone is an intracellular pH-sensitive fluorescent indicator and blood-brain barrier probe.[8]
Umbelliferone derivatives have antimicrobial properties, and has anti-mutagenic effects induced by 4-nitroquinoline 1-oxide or UV irradiation in E. Coli.[9,10]
References:
[1] Ramesh B, Viswanathan P, Pugalendi K V. Eur J Pharmacol, 2007, 566(1-3):231–9.
[2] Ramesh B, Pugalendi K V. J Med Food, 2006, 9(4):562-6.
[3] Pillai S P, Menon S R, Mitscher L A, et al. J Nat Prod, 1999, 62(10):1358-62.
[4] Rajbir Singh, Bikram Singh, Sukhpreet Singh,et al. Food Chem, 2010, 120(3):825-30.
[5] Kumar P, Singh V K, Singh D K. Phytother Res, 2009, 23(2):172–7.
[6] Fan G J, Mar W, Man K P, et al. Bioorg Med Chem Lett, 2001, 11(17):2361-3.
[7] Vasconcelos J F, Teixeira M M, Barbosa-Filho J M, et al. Eur J Pharm, 2009, 609:126-31.
[8] Jr S T, Anderson R E. J Neurophysiol, 1980, 44(1):60-75.
[9] Jurd L, King A D, Mihara K. Phytochemistry, 1971, 10(12):2965-70.
[10] Ohta T, Watanabe K, Moriya M, et al. Mutation Research/fundamental & Molecular Mechanisms of Mutagenesis, 1983, 117(1-2):135-8.
[11] Liu L X, Zhao T, Sun L X. Journal of Shenyang Pharmaceutical University, 2010, 27(7):563-6.
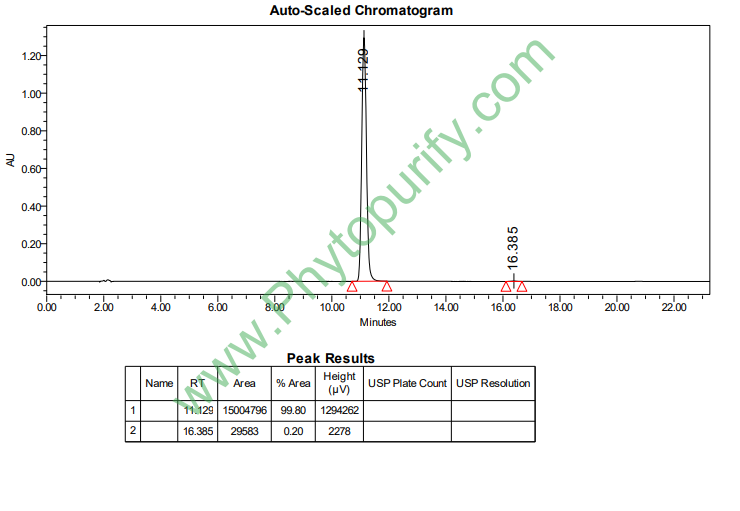
HPLC of 7-Hydroxycoumarin