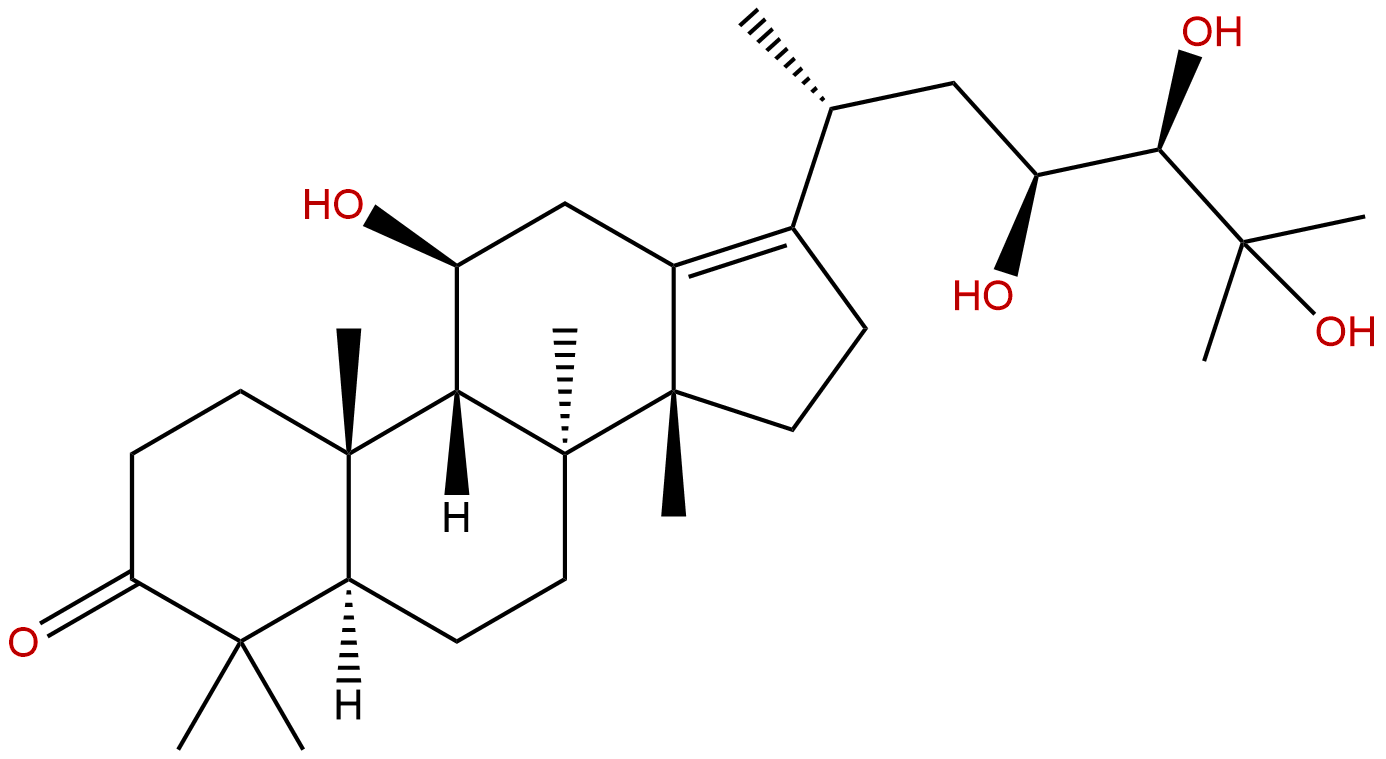
Alisol ACAS No.:19885-10-0
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0137 |
| Formula: | C30H50O5 |
| Mol Weight: | 490.725 |
Product name: Alisol A
Synonym name:
Catalogue No.: BP0137
Cas No.: 19885-10-0
Formula: C30H50O5
Mol Weight: 490.725
Botanical Source: Alisma orientale(Sam.) Juzep.
Physical Description:
Type of Compound: Triterpenoids
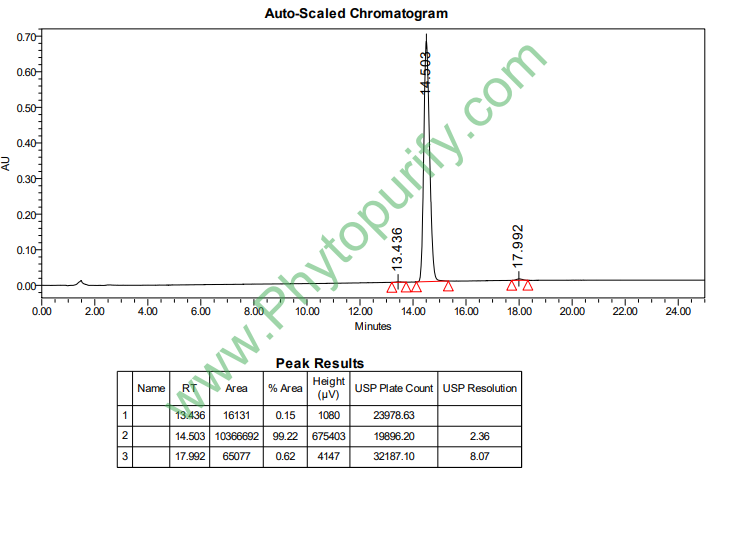
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Alisol A may be an autophagic inducer, it has anti-cancer activity, it presents inhibitory effects on cancer cell lines tested(HepG2, MDA-MB-231, and MCF-7 cells).
References:
Phytochemistry. 2016 Nov;131:150-157.
Structures and biological activities of the triterpenoids and sesquiterpenoids from Alisma orientale.
Sixteen triterpenoids and nine sesquiterpenoids were isolated from the rhizome of Alisma orientale. Structures of 16-oxo-11-anhydroAlisol A 24-acetate, 13β,17β-epoxy-24,25,26,27-tetranor-Alisol A 23-oic acid, 1αH,5αH-guaia-6-ene-4β,10β-diol, and alisguaiaone were elucidated by comprehensive spectroscopic data analysis.
METHODS AND RESULTS:
The cytotoxic, antibacterial, antifungal, anti-inflammatory, and α-glucosidase inhibitory activities of isolated terpenoids were evaluated. Triterpenoids Alisol A, Alisol A 24-acetate, 25-O-ethylAlisol A, 11-deoxyAlisol A, alisol E 24-acetate, alisol G, alisol B 23-acetate and sesquiterpenoids 1αH,5αH-guaia-6-ene-4β,10β-diol, 10-hydroxy-7,10-epoxysalvialane exhibited cytotoxicities against the three tested human cancer cell lines with IC50 values ranging from 11.5 ± 1.7 μM to 76.7 ± 1.4 μM. Triterpenoids Alisol A, 25-O-ethylAlisol A, 11-deoxyAlisol A, alisol E 24-acetate, alisol G, and 25-anhydroalisol F showed antibacterial activities against the Gram-positive strains Bacillus subtilis and Staphylococcus aureus with MIC values of 12.5-100 μg/mL. Sesquiterpenoid 4β,10β-dihydroxy-1αH,5βH-guaia-6-ene exhibited antibacterial activity against B. subtilis with an MIC value of 50 μg/mL, and 10-hydroxy-7,10-epoxysalvialane exhibited activity against S. aureus with an MIC value of 100 μg/mL. Compounds 16-oxo-11-anhydroAlisol A 24-acetate, alisol F, 25-anhydroalisol F, and alisguaiaone exhibited inhibitory effects on lipopolysaccharide-induced NO production in RAW 264.7 macrophage cells. None of the compounds showed obvious inhibitory activity against α-glucosidase.
HPLC of Alisol A