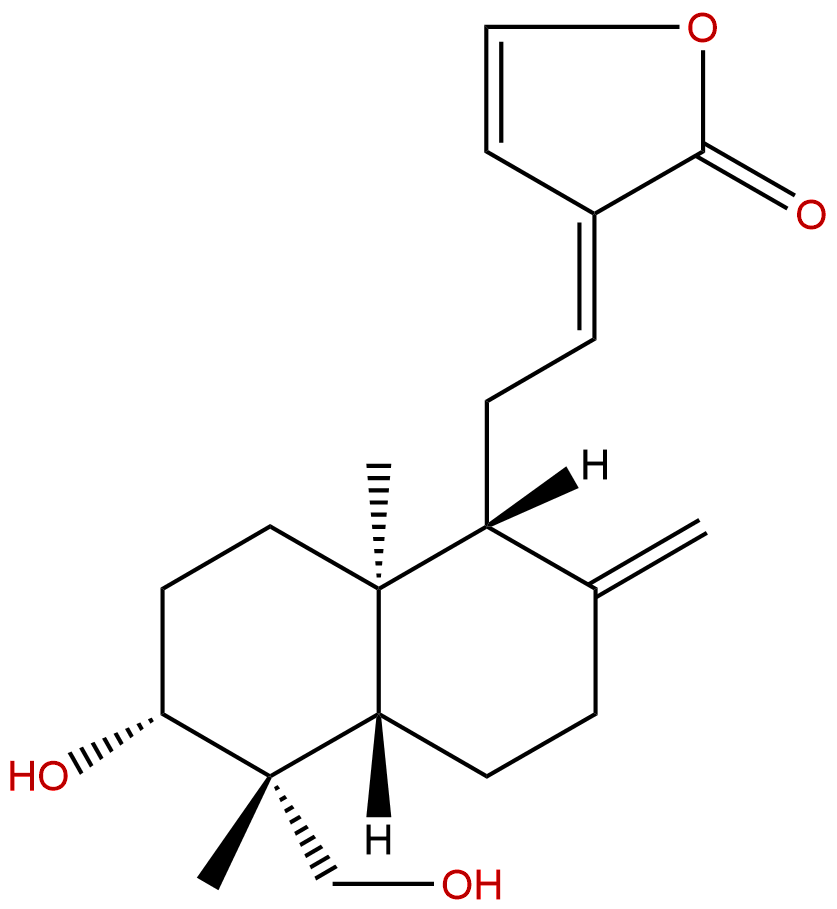
DehydroandrographolideCAS No.:134418-28-3
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0459 |
| Formula: | C20H28O4 |
| Mol Weight: | 332.44 |
Product name: Dehydroandrographolide
Synonym name:
Catalogue No.: BP0459
Cas No.: 134418-28-3
Formula: C20H28O4
Mol Weight: 332.44
Botanical Source: Andrographis Herba
Physical Description:
Type of Compound: Diterpenoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Dehydroandrographolide is a novel TMEM16A inhibitor and possesses multiple pharmacological activities, including anti-inflammation, anti-cancer, anti-bacterial, anti-virus and anti-hepatitis activity. It possesses activity against HBV DNA replication with IC50 values of 22.58 uM and low SI values of 8.7 ; it can alleviate oxidative stress in LPS-induced acute lung injury possibly by inactivating iNOS.
References:
DARU., 2015, 23(1):1-7.
Dehydroandrographolide enhances innate immunity of intestinal tract through up-regulation the expression of hBD-2.
Dehydroandrographolide (DA) is one of major active components in the well-known oriental herbal medicine Andrographis paniculata (Burm.f) Nees which belongs to the Acanthaceae family. DA is used for the treatment of infections in China. However, DA has not been found to significantly inhibit bacterial and viral growth directly. The current study investigates the effect of DA on the expression of human β -defensin-2 (hBD-2) in human intestinal epithelial cells and the possible signaling pathways.
METHODS AND RESULTS:
Human intestinal epithelial HCT-116 cells were incubated with 1-100 μM DA for 2-24 h. RT-PCR and Western blot were used to assess the expression of hBD-2. The specific inhibitors were used and the levels of phosphorylation of signaling molecules were detected for dissecting the signaling pathways leading to the induction of hBD-2. MTT assay showed there was no obvious cytotoxicity for HCT-116 cells by 1-100 μM DA treatment. RT-PCR and Western blot assays showed that DA (1-100 μM) could up-regulate the expression of hBD-2, and the effect lasted longer than 24 h. By using SB203580 and SB202190 (inhibitors of p38), the enhancement of hBD-2 expression were significantly attenuated. However, inhibitor of ERK and inhibitor of JNK could not block the effect of DA. Furthermore, Western blot found activation of p38 but not ERK and JNK in DA-treated HCT-116 cells.
CONCLUSIONS:
The results suggested that DA enhanced innate immunity of intestinal tract by up-regulating the expression of hBD-2 through the p38 MAPK pathways.
Bioorg Med Chem Lett. 2014 May 15;24(10):2353-9.
Synthesis, structure-activity relationships and biological evaluation of dehydroandrographolide and andrographolide derivatives as novel anti-hepatitis B virus agents.
Dehydroandrographolide and andrographolide, two natural diterpenoids isolated from Andrographis paniculata possessed activity against HBV DNA replication with IC50 values of 22.58 and 54.07μM and low SI values of 8.7 and 3.7 in our random assay.
METHODS AND RESULTS:
Consequently, 48 derivatives of Dehydroandrographolide and andrographolide were synthesized and evaluated for their anti-HBV properties to yield a series of active derivatives with lower cytotoxicity, including 14 derivatives against HBsAg secretion, 19 derivatives against HBeAg secretion and 38 derivatives against HBV DNA replication. Interestingly, compound 4e could inhibit not only HBsAg and HBeAg secretions but also HBV DNA replication with SI values of 20.3, 125.0 and 104.9. Furthermore, the most active compound 2c with SI value higher than 165.1 inhibiting HBV DNA replication was revealed with the optimal logP value of 1.78 and logD values.
CONCLUSIONS:
Structure-activity relationships (SARs) of the derivatives were disclosed for guiding the future research toward the discovery of new anti-HBV drugs.
Chin J Integr Med. 2014 Dec 9.
Potassium Dehydroandrographolide Succinate Injection for the treatment of child epidemic parotitis: A systematic review and meta-analysis.
To systematically evaluate the clinical efficacy and safety of Potassium Dehydroandrographolide Succinate Injection (PDSI) in the treatment of child epidemic parotitis (EP).
METHODS AND RESULTS:
Randomized controlled trials (RCTs) regarding Potassium Dehydroandrographolide Succinate Injection in the treatment of child EP were searched in China National Knowledge Infrastructure, Wanfang Database, Chinese Biomedical Literature Database, PubMed, and Cochrane Library from inception to July 30, 2013. Two reviewers independently retrieved RCTs and extracted information. The Cochrane risk of bias method was used to assess the quality of included studies, and a metaanalysis was conducted with RevMan 5.2 software. A total of 11 studies with 818 participants were included. The quality of the studies was generally low, among which only one study mentioned the random method. The meta-analysis indicated that Potassium Dehydroandrographolide Succinate Injection was more effective than the conventional therapy with Western medicine for EP in the outcomes of the total effective rate [relative risk (RR)=1.23, 95% confidence interval (CI) [1.14, 1.33], P<0.01], the time of temperature return to normal, the time of detumescence [mean difference (MD)=-2.10, 95% CI [-2.78, -1.41], P<0.01], and the incidence of complications (RR=0.14, 95% CI [0.03, 0.72], P=0.02). There were 6 adverse drug reactions (ADRs) in this systematic review, 2 of which were mainly represented rash and diarrhea in the experiment group, while another 4 ADRs occurred in the control group.
CONCLUSIONS:
Based on the systematic review, Potassium Dehydroandrographolide Succinate Injection was effectiveness and relatively safety in the treatment of child EP. But further rigorously designed trials are warranted to determine its effectiveness.
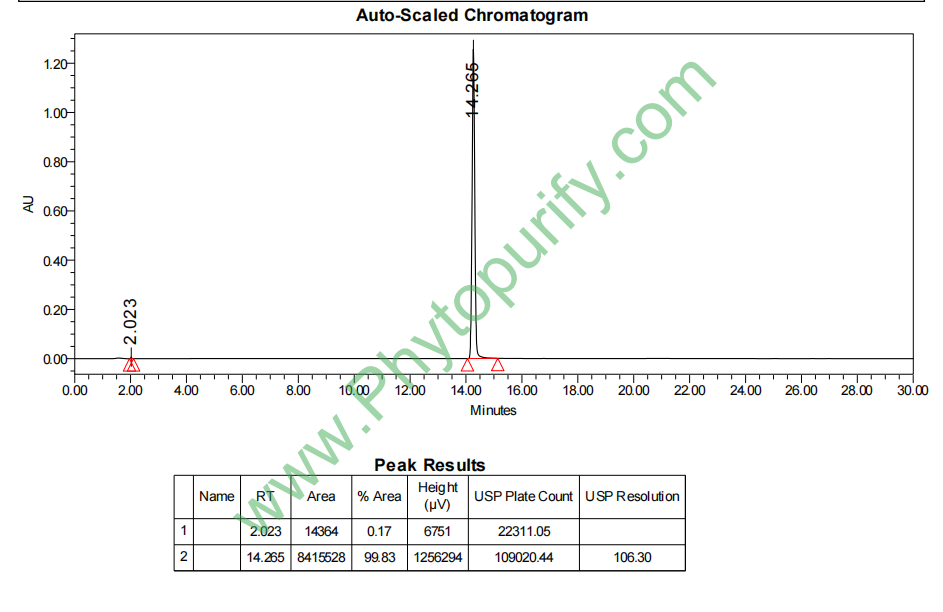
HPLC of Dehydroandrographolide