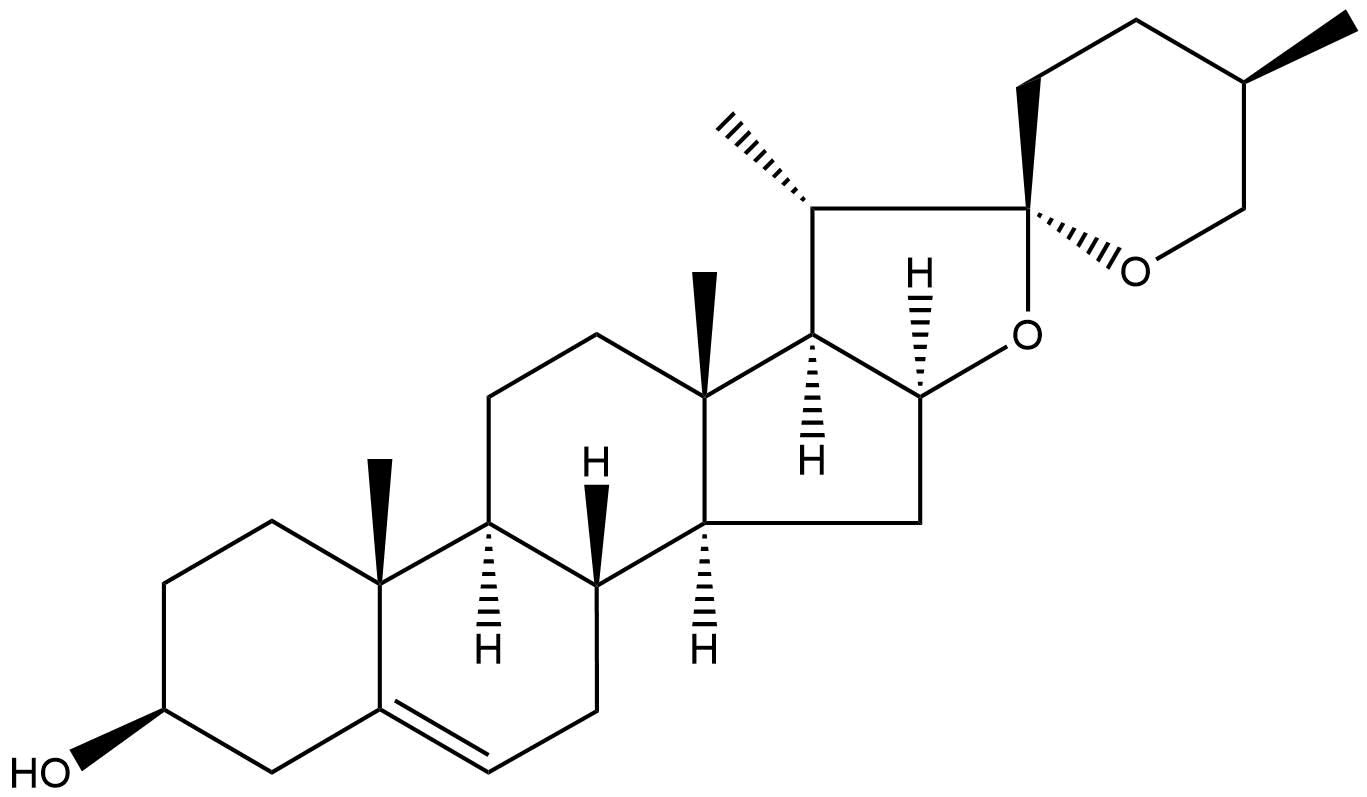
Diosgenin Descrtption
Product name: Diosgenin
Synonym name: Nitogenin; Dioscorea sapogenin
Catalogue No.: BP0504
Cas No.: 512-04-9
Formula: C27H42O3
Mol Weight: 414.63
Botanical Source: Sapogenin from Dioscorea spp., Solanum spp. Trillium erectum, Balanites aegyptiaca, Aletris farinosa, Clintonia spp. and many other plants
Physical Description: Powder
Type of Compound: Steroids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams, up to kilograms
Inquire for bulk scale.
Descriptions:
Diosgenin, a steroidal saponin present in fenugreek (Trigonella foenum graecum) and other plants, has been shown to suppress inflammation, inhibit proliferation, and induce apoptosis in a variety of tumor cells; it suppresses proliferation, inhibits invasion, and suppresses osteoclastogenesis through inhibition of NF-kappaB-regulated gene expression and enhances apoptosis induced by cytokines and chemotherapeutic agents.[1]
Diosgenin alters cell cycle distribution and induced apoptosis in the human osteosarcoma 1547 cell line, it induces apoptosis is caspase-3 dependent with a fall of mitochondrial membrane potential, nuclear localization of AIF and poly (ADP-ribose) polymerase cleavage, diosgenin treatment also induces p53 activation and cell cycle arrest in the different cell lines studied.[2]
Diosgenin and L-deprenyl can increase vulnerability of ApoE4 neurons to HIV proteins and opiates, opiate abusers with HIV infection and the ApoE4 allele may be at increased risk of developing dementia, thus they may have therapeutic potential in this population.[3]
Diosgenin can induce apoptosis, cell cycle arrest and COX activity in osteosarcoma cells.[4]
Diosgenin can induce the expression of Src homology 2 phosphatase 2 (SH-PTP2) that correlated with downregulation of constitutive STAT3 activation, also downregulate the expression of various STAT3-regulated gene products, inhibit proliferation and potentiated the apoptotic effects of paclitaxel and doxorubicin, suggest that it is a novel blocker of the STAT3 activation pathway, with a potential role in the treatment of HCC and other cancers.[5]
Diosgenin significantly inhibits tumor growth in both MCF-7 and MDA-231 xenografts in nude mice by inhibiting pAkt expression and Akt kinase activity without affecting PI3 kinase levels, resulting in the inhibition of its downstream targets, NF-kappaB, Bcl-2, survivin and XIAP, thus, it might prove to be a potential chemotherapeutic agent for the treatment of breast cancer .[6]
Diosgenin has a hypocholesterolemic effect by suppressing cholesterol absorption and increasing cholesterol secretion, it could be a very useful compound to control hypercholesterolemia by both improving the lipid profile and modulating oxidative stress.[7]
References:
[1] Shishodia S, Aggarwal B B. Oncogene, 2005, 25(10):1463-73.
[2] CecileCORBIERE, BertrandLIAGRE, FarajTERRO. Cell Res, 2004, 14(3):188-96.
[3] Turchan-Cholewo J, Liu Y, Gartner S, et al. Neurobiol Dis, 2006, 23(1):109-19.
[4] Moalic S, Liagre B, Corbière C, et al. Febs Lett, 2001, 506(3):225-30.
[5] Li F, Fernandez P P, Rajendran P, et al. Cancer Lett, 2010, 292(2):197-207.
[6] Srinivasan S, Koduru S, Kumar R, et al. Int J Cancer, 2009, 125(4):961-7.
[7] Son I S, Ji H K, Sohn H Y, et al. Biosci Biotech Bioch, 2014, 71(12):3063-71.
[8] Warke V B, Deshmukh T A, Patil V R. Asian J Pharm Clin Res 2011, 4:126-8.