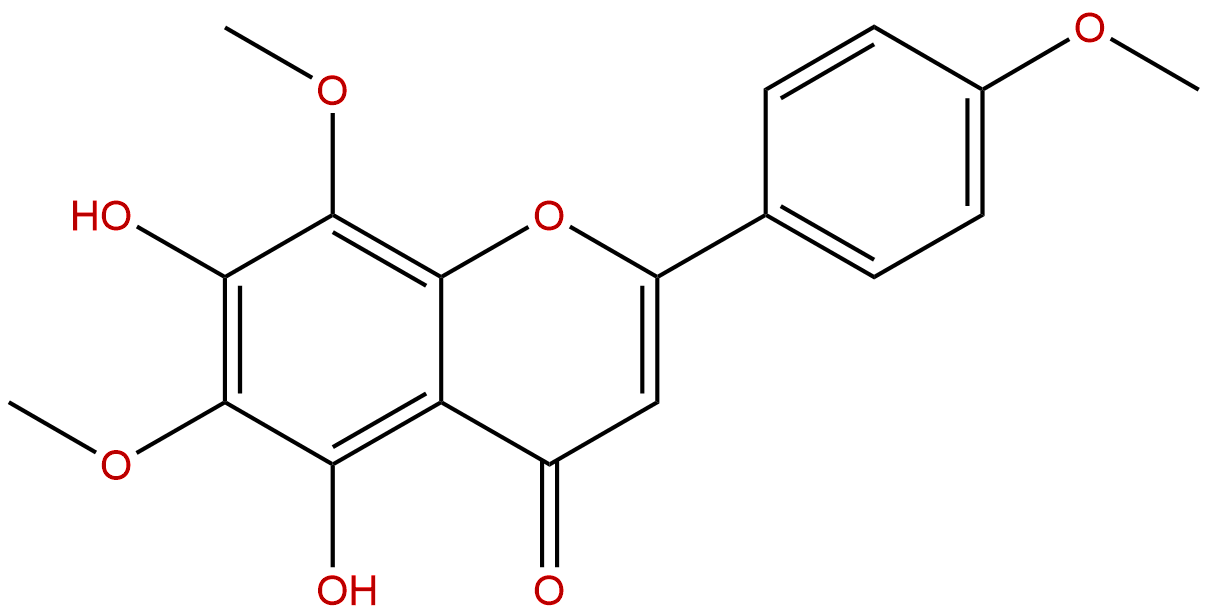
LysionotinCAS No.:10176-66-6
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0906 |
| Formula: | C18H16O7 |
| Mol Weight: | 344.319 |
Synonym name: Nevadensin; Ethoxychelerythrine; 152743-19-6
Catalogue No.: BP0906
Cas No.: 10176-66-6
Formula: C18H16O7
Mol Weight: 344.319
Botanical Source:
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Description:
Nevadensin is an important herb-based constituent inhibiting estragole bioactivation. Nevadensin protects against a methyleugenol-induced marker of hepatocarcinogenicity in male F344 rat. It also exhibits inhibition activity against Mycobacterium tuberculosis, with equal MIC value of 200 microg/mL.
References:
Food Chem Toxicol. 2014 Dec;74:28-34.
The natural basil flavonoid nevadensin protects against a methyleugenol-induced marker of hepatocarcinogenicity in male F344 rat.
The alkenylbenzene methyleugenol occurs naturally in a variety of spices and herbs, including basil, and their essential oils. At high dose levels methyleugenol induces hepatocarcinogenicity in rodents following bioactivation to 1'-sulfooxymethyleugenol which forms DNA adducts.
METHODS AND RESULTS:
This study investigated whether the inhibitory effect of the basil flavonoid Nevadensin on sulfotransferase (SULT)-mediated bioactivation of methyleugenol observed in vitro would also be reflected in a reduction of DNA adduct formation and a reduction in an early marker for liver carcinogenesis in an 8-week rat study. Co-exposure to methyleugenol and Nevadensin orally resulted in a significant inhibition of liver methyleugenol DNA adduct formation and in inhibition of hepatocellular altered foci induction, representing indicators for initiation of neoplasia.
CONCLUSIONS:
These results suggest that tumor formation could be lower in rodent bioassays when methyleugenol would be dosed in a matrix containing SULT inhibitors such as Nevadensin compared to experiments using the pure methyleugenol.
Arch Pharm Res. 2003 Oct;26(10):816-20.
Antimycobacterial and antioxidant flavones from Limnophila geoffrayi.
The chloroform extract of the aerial part of Limnophila geoffrayi showed antimycobacterial and antioxidant activities.
METHODS AND RESULTS:
Bioassay-guided fractionation has led to the isolation of the flavones Nevadensin (5,7-dihydroxy-6,8,4'-trimethoxyflavone, 1) and isothymusin (6,7-dimethoxy-5,8,4'-trihydroxyflavone, 2). Both compounds 1 and 2 exhibited inhibition activity against Mycobacterium tuberculosis, with equal MIC value of 200 microg/mL. Only compound 2 exhibited antioxidant activity against the radical scavenging ability of DPPH, with the IC50 value of 7.7 microg/mL.
CONCLUSIONS:
The crude hexane, chloroform and methanol extracts as well as the pure compounds 1 and 2 did not exhibit mutagenic activity in the Bacillus subtilis recassay.
Toxicol Appl Pharmacol. 2010 Jun 1;245(2):179-90.
Identification of nevadensin as an important herb-based constituent inhibiting estragole bioactivation and physiology-based biokinetic modeling of its possible in vivo effect.
Estragole is a natural constituent of several herbs and spices including sweet basil. In rodent bioassays, estragole induces hepatomas, an effect ascribed to estragole bioactivation to 1'-sulfooxyestragole resulting in DNA adduct formation.
METHODS AND RESULTS:
The present paper identifies Nevadensin as a basil constituent able to inhibit DNA adduct formation in rat hepatocytes exposed to the proximate carcinogen 1'-hydroxyestragole and Nevadensin. This inhibition occurs at the level of sulfotransferase (SULT)-mediated bioactivation of 1'-hydroxyestragole. The Ki for SULT inhibition by Nevadensin was 4 nM in male rat and human liver fractions. Furthermore, Nevadensin up to 20 microM did not inhibit 1'-hydroxyestragole detoxification by glucuronidation and oxidation. The inhibition of SULT by Nevadensin was incorporated into the recently developed physiologically based biokinetic (PBBK) rat and human models for estragole bioactivation and detoxification. The results predict that co-administration of estragole at a level inducing hepatic tumors in vivo (50mg/kg bw) with Nevadensin at a molar ratio of 0.06, representing the ratio of their occurrence in basil, results in almost 100% inhibition of the ultimate carcinogen 1'-sulfooxyestragole when assuming 100% uptake of Nevadensin. Assuming 1% uptake, inhibition would still amount to more than 83%. Altogether these data point at a Nevadensin-mediated inhibition of the formation of the ultimate carcinogenic metabolite of estragole, without reducing the capacity to detoxify 1'-hydroxyestragole via glucuronidation or oxidation.
CONCLUSIONS:
These data also point at a potential reduction of the cancer risk when estragole exposure occurs within a food matrix containing SULT inhibitors compared to what is observed upon exposure to pure estragole.
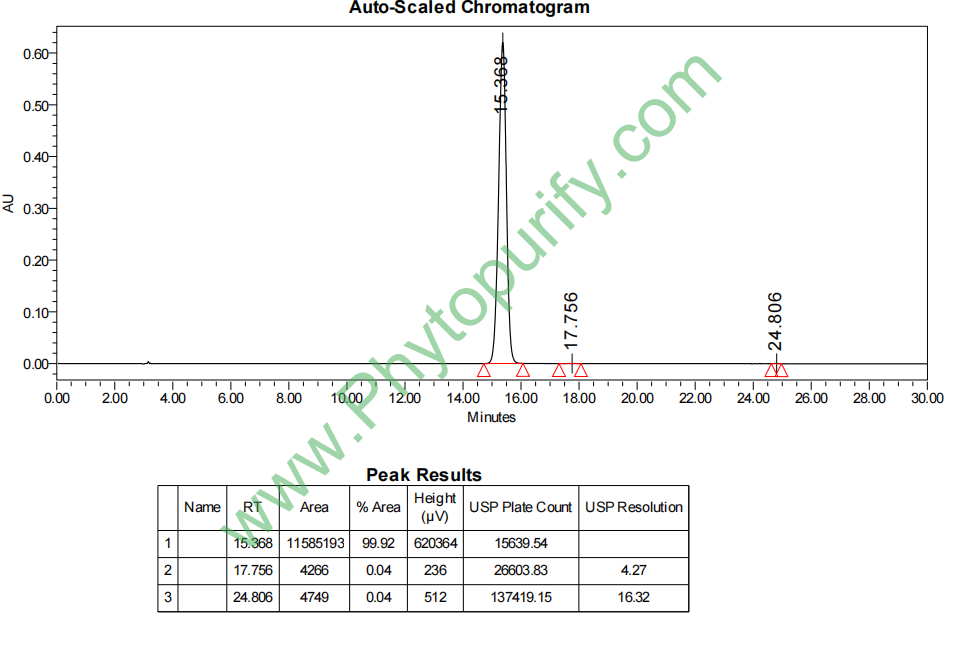
HPLC of Lysionotin