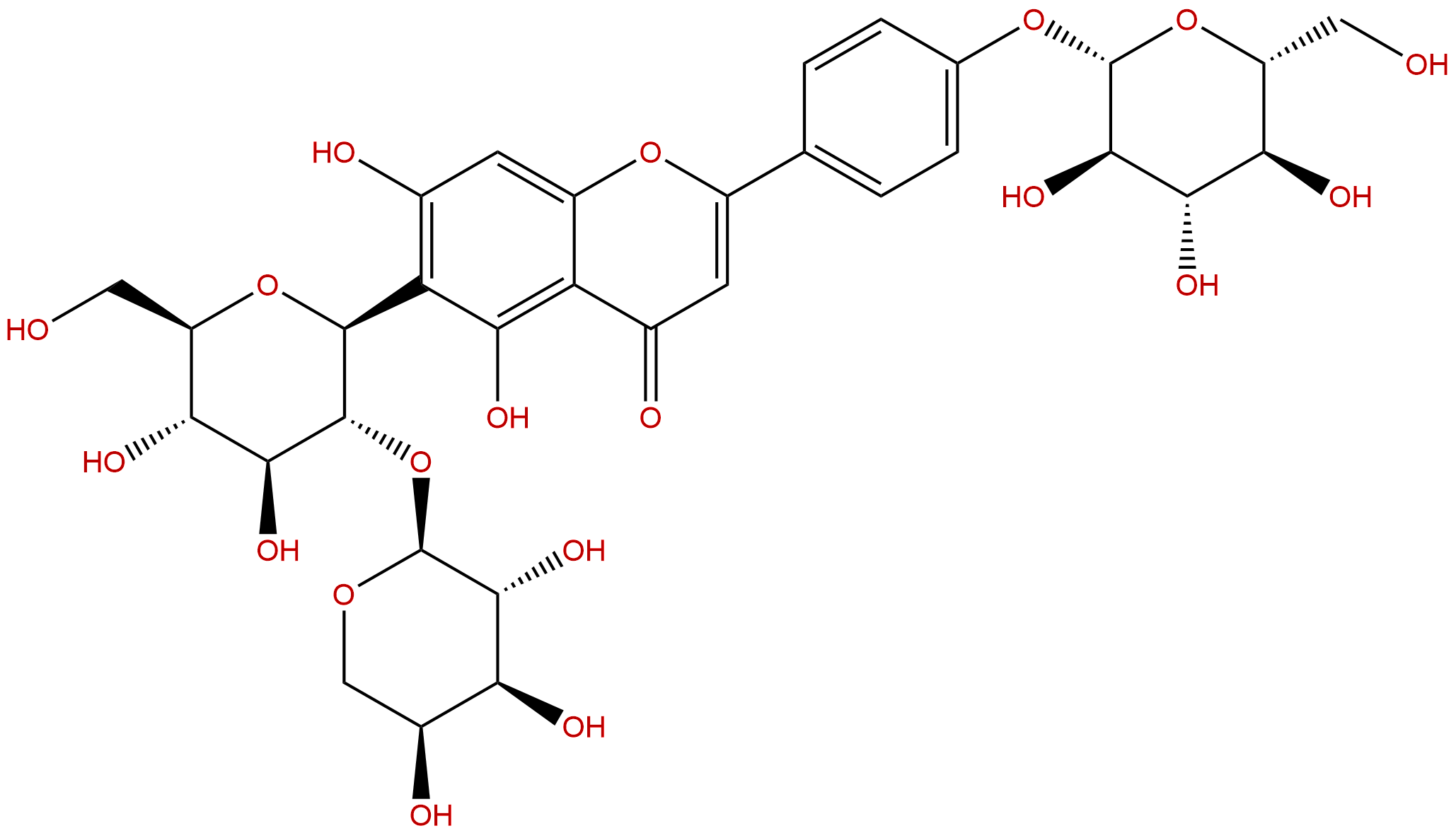
VaccarinCAS No.:53452-16-7
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1428 |
| Formula: | C32H38O19 |
| Mol Weight: | 726.637 |
Product name: Vaccarin
Synonym name:
Catalogue No.: BP1428
Cas No.: 53452-16-7
Formula: C32H38O19
Mol Weight: 726.637
Botanical Source: Vaccaria segetalis(Neck.)Garcke
Physical Description:
Type of Compound: Flavonoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Vaccarin exhibits extensive biological activities including vascular endothelial cell protective effects, it can significantly promote neovascularization by enhancing protein expression of p-Akt , p‑Erk, and CD31, and selectively protect vascular endothelium from dysfunction induced by H2O2.
References:
Mol Med Rep. 2015 Jul;12(1):1131-6.
Vaccarin promotes endothelial cell proliferation in association with neovascularization in vitro and in vivo.
Angiogenesis is a major pathological component of several diseases, including traumatic vascular disease and coronary heart disease. The purpose of the present study was to determine the effects of Vaccarin on endothelial cell migration and neovascularization, which are important and necessary components of wound healing.
METHODS AND RESULTS:
The present study investigated and confirmed neovascularization induced by Vaccarin in vitro and in vivo. In vitro, the effects of Vaccarin (1.08 and 2.15 µM) on proliferation, migration and tube formation of human microvascular endothelial cells (HMEC)-1 were evaluated via sulforhodamine B assay and migration and tube formation assay, respectively. Furthermore, a mouse Matrigel plus model was used to detect capillary-like tube structures in vivo. Immunohistochemistry was used to detect the protein expression of cluster of differentiation 31 (CD31), p-AKT and p-extracellular-signal-regulated kinases (Erk). Vaccarin significantly promoted HMEC-1 proliferation and migration and tube formation of HMEC-1 at a dose of 2.15 µM. In vivo, Vaccarin delivered by daily oral administration significantly improved epidermal growth factor-induced angiogenesis in an intradermal inoculation mouse model.
CONCLUSIONS:
The mouse Matrigel model study also revealed that Vaccarin significantly promoted neovascularization via detection of CD31 levels and enhanced protein expression of p-Akt and p‑Erk. In addition, Vaccarin also promoted expression of CD31.
Int J Mol Med. 2015 Jan;35(1):135-42.
Vaccarin attenuates the human EA.hy926 endothelial cell oxidative stress injury through inhibition of Notch signaling.
Endothelial cell injury is an essential component of atherosclerosis and hypertension. Atherosclerosis and other macrovascular diseases are the most common complications of diabetes. Vaccarinis a major flavonoid glycoside in Vaccariae semen, and is expected to be useful in the treatment of vascular diseases. The aim of the present study was to evaluate the possible effects of Vaccarinin human umbilical vein endothelial cells (EA.hy926) induced by hydrogen peroxide (H2O2) and its underlying mechanism in the prevention and treatment of H2O2 injury.
METHODS AND RESULTS:
In this study, the EA.hy926 cells were exposed to 250, 500 and 1000 µM H2O2 for 2 and 4 h in the absence or presence of Vaccarin, and the cell injury induced by H2O2 was examined via SRB. Cell migratory ability, lactate dehydrogenase (LDH) leakage, malondialdehyde (MDA) levels and decreasing superoxide dismutase (SOD) activity were evaluated by the wound healing assay and corresponding assay kits. Cell apoptosis was detected by flow cytometry with Annexin V-fluorescein isothiocyanate/propidium iodide Apoptosis Detection kit and Hoechst staining. Furthermore, western blot detected the protein expressions of Notch1, Hes1 and caspase-3. Following treatment with H2O2, it was found that H2O2 stimulated cell injury in a dose-dependent manner, including reducing cell viability and cell migratory ability, increasing LDH leakage and MDA levels, and decreasing SOD activity. H2O2 further accelerated cell apoptosis via activation of Notch1 and the downstream molecule Hes1. Preincubation with Vaccarin was found to protect EA.hy926 cells from H2O2-induced cell oxidative stress injury, which promoted cell viability and cell migratory ability, inhibited the level of LDH and MDA, but enhanced the activity of SOD. In particular, in addition to downregulation Notch signaling, Vaccarin treatments also downregulated caspase-3, a cell apoptotic pathway-related protein.
CONCLUSIONS:
These findings indicated that Vaccarin may be able to selectively protect vascular endothelium from dysfunction induced by H2O2.
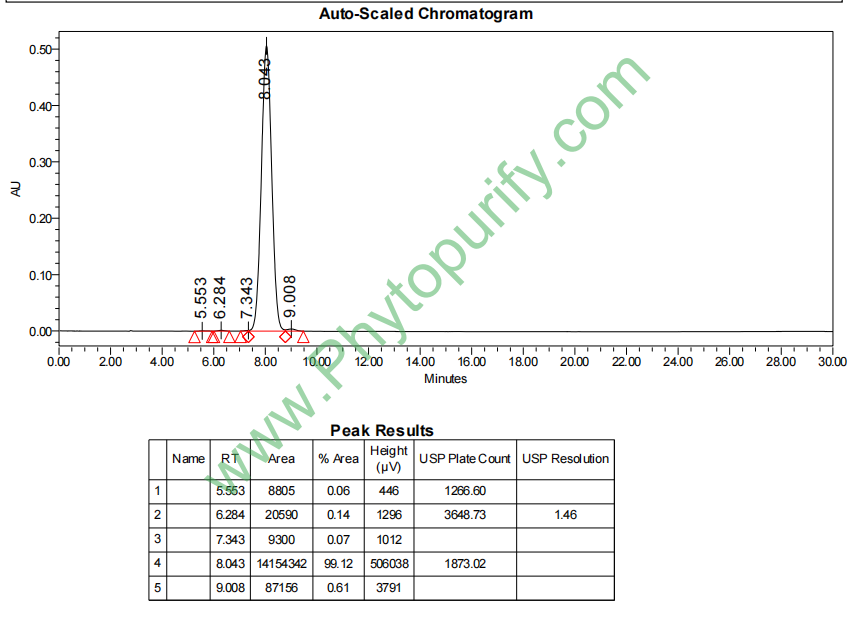
HPLC of Vaccarin