
BufoteniniumCAS No.:60657-23-0
|
||||||||||
 |
|
|
||||||||
|
|
||||||||||

| Catalogue No.: | BP4266 |
| Formula: | C13H19N2O+ |
| Mol Weight: | 219.307 |
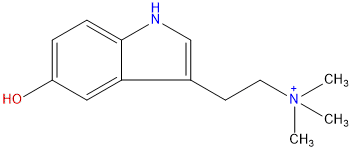
Product name: Bufoteninium
Synonym name: 5-Hydroxy-N,N,N-trimethyl-1H-indole-3-ethanaminium
Catalogue No.: BP4266
Cas No.: 60657-23-0
Formula: C13H19N2O+
Mol Weight: 219.307
Botanical Source: Bufonis Venenum
Type of Compound: Alkaloids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
Cinobufotenine is a potent stimulant of parasympathetic ganglia and its effect are competitively antagonized by hexamethonium.
References:
Br J Pharmacol. 1980 Aug;69(4):597-600.
A comparison of cinobufotenine (the quaternary derivative of 5-HT) and some related compounds with coryneine (the quaternary derivative of dopamine) on the frog rectus, guinea-pig ileum and rat fundus strip preparations.
METHODS AND RESULTS:
1 Coryneine is 2.7 times as active as Cinobufotenine on the frog rectus but on the guinea-pig ileum Cinobufotenine is 1.5 times as active as coryneine. Cinobufotenine is a potent stimulant of parasympathetic ganglia and its effect are competitively antagonized by hexamethonium. 2 The effects of pH on activity relative to a standard whose ionisation is constant (Me4+N or the trimethylammonium analogue of tryptamine) are consistent with the phenate form being weaker than the phenolic form but the changes are smaller than with coryneine because Cinobufotenine is a weaker acid.
CONCLUSIONS:
3 The hydroxyl group makes a large contribution to activity. Cinobufotenine is 9 times as active as the analogue without a hydroxyl on the frog rectus and 12 times as active as it on the ileum. The 5-methoxy analogue is an antagonist on the frog rectus and a very weak partial agonist on the ileum. 4 Cinobufotenine and the quaternary derivative of tryptamine have less than one-thousandth of the activity of 5-hydroxytryptamine on the rat fundus strip.