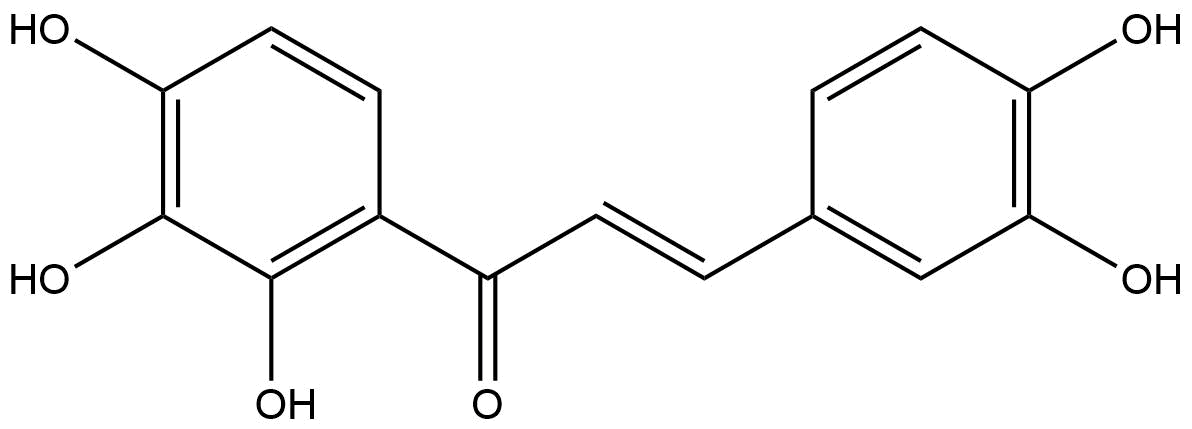
OkaninCAS No.:484-76-4
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP2137 |
| Formula: | C15H12O6 |
| Mol Weight: | 288.255 |
Product name: Okanin
Synonym name: 2',3,3',4,4'-Pentahydroxychalcone
Catalogue No.: BP2137
Cas No.: 484-76-4
Formula: C15H12O6
Mol Weight: 288.255
Botanical Source: Coreopsis tinctoria Nutt.
Type of Compound:
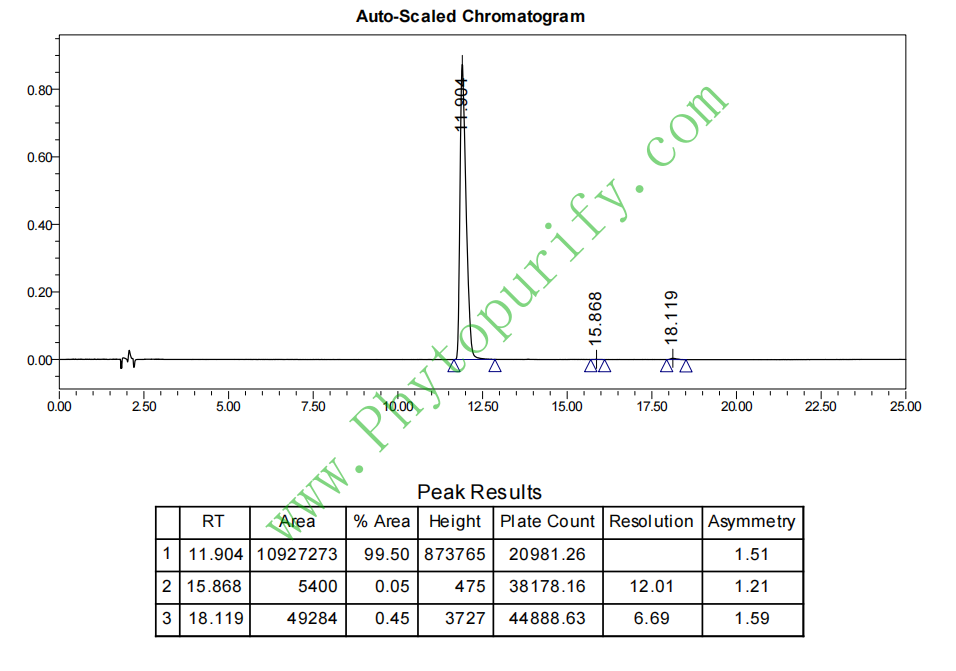
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
1. Okanin shows significant activities on both linoleic acid peroxidation inhibition and DPPH radical scavenging effect.
2. Okanin shows significant protective effects against tacrine-induced cytotoxicity in human liver-derived Hep G2 cells with the EC50 value of 29.8±1.1 uM.
3. Okanin can inhibit nitric oxide production and inducible nitric oxide synthase expression via nuclear factor-erythroid 2-related factor 2-dependent heme oxygenase-1 expression in lipopolysaccharide-activated macrophages.
4. Okanin attenuates LPS-induced microglial activation through inhibition of the TLR4/NF-κB signaling pathways, suggests that okanin may have potential as a nutritional preventive strategy for neurodegenerative disorders.
References:
Korean Journal of Pharmacognosy, 2009 , 40 (4) :345-50.
Antioxidative and hepatoprotective effect of compounds from the flowers of bidens Bipinnata L.
METHODS AND RESULTS:
Activity-guided fractionation of the EtOH extract of flowers of Bidens bipinnata L. have been furnished five flavonoids, sulfuretin(1), butein(2), 7,8,3′,4′-tetrahydroxyflavanone(3), maritimetin(4) and Okanin(5). All of the compounds showed significant activities on both linoleic acid peroxidation inhibition and DPPH radical scavenging effect. The evaluation for protective effect of isolated compounds against tacrine-induced cytotoxicity in human liver-derived Hep G2 cells was conducted. Compounds 1, 3, 4 and 5 showed significant protective effects with the EC50 values of 36.1±0.9, 23.3±0.7, 41.0±1.0 and 29.8±1.1 μM, respectively.
CONCLUSIONS:
Silybin, one of the well-known hepatoprotective agents, used as a positive control, and also showed protective effect with an EC50 value of 84.3±0.7 μM.
J Clin Biochem Nutr. 2012 Jan; 50(1): 53–58.
Okanin, a chalcone found in the genus Bidens, and 3-penten-2-one inhibit inducible nitric oxide synthase expression via heme oxygenase-1 induction in RAW264.7 macrophages activated with lipopolysaccharide
Okanin is one of the most abundant chalcones found in the genus Bidens (Asteraceae) that is used as various folk medications in Korea and China for treating inflammation.
METHODS AND RESULTS:
Here, we found that Okanin (possessing the α-β unsaturated carbonyl group) induced heme oxygenase-1 expression via nuclear factor-erythroid 2-related factor 2 activation in RAW264.7 macrophages. 3-Penten-2-one, of which structure, as in Okanin, possesses the α-β unsaturated carbonyl group, also induced nuclear factor-erythroid 2-related factor 2-dependent heme oxygenase-1 expression, while both 2-pentanone (lacking a double bond) and 2-pentene (lacking a carbonyl group) were virtually inactive. In lipopolysaccharide-activated RAW264.7 macrophages, both Okanin and 3-penten-2-one inhibited nitric oxide production and inducible nitric oxide synthase expression via heme oxygenase-1 expression.
CONCLUSIONS:
Collectively, our findings suggest that by virtue of its α-β unsaturated carbonyl functional group, Okanin can inhibit nitric oxide production and inducible nitric oxide synthase expression via nuclear factor-erythroid 2-related factor 2-dependent heme oxygenase-1 expression in lipopolysaccharide-activated macrophages.
HPLC of Okanin