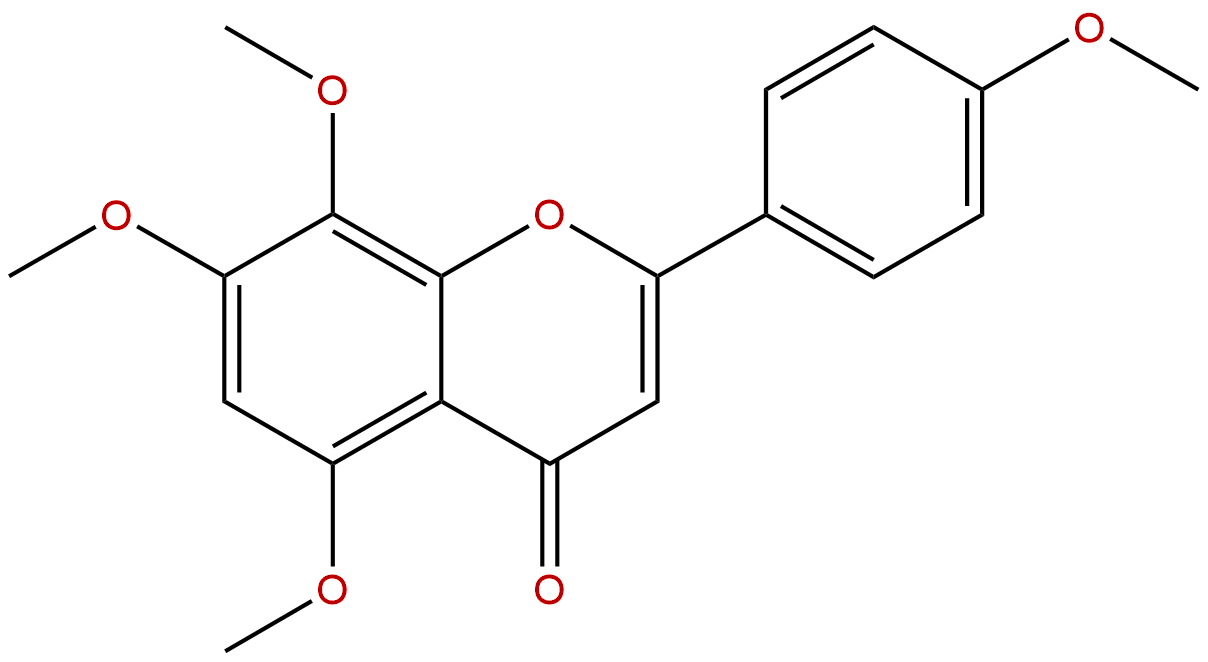
6-DemethoxytangeretinCAS No.:6601-66-7
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1787 |
| Formula: | C19H18O6 |
| Mol Weight: | 342.347 |
Product name: 6-Demethoxytangeretin
Synonym name: 4',5,7,8-Tetramethoxyflavone; 6-Demethoxytangeritin; Tetra-O-methylisoscutellarein
Catalogue No.: BP1787
Cas No.: 6601-66-7
Formula: C19H18O6
Mol Weight: 342.347
Botanical Source: Citrus sinensis
Physical Description:
Type of Compound: Flavonoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
The product could be supplied from milligrams to grams. Inquire for bulk scale.
We provide solution to improve the water-solubility of compounds, thereby facilitating the variety of activity tests and clinic uses.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
6-Demethoxytangeretin has anti-inflammatory activity, it also can suppress production and gene expression of interleukin-6 in human mast cell-1 via anaplastic lymphoma kinase and mitogen-activated protein kinase pathways. 6-Demethoxytangeretin can suppress the interleukin 1 (IL-1) induced production of proMMP-9/progelatinase B in rabbit synovial cells in a dose dependent manner (<64 microM).
References:
Biol Pharm Bull. 2013;36(10):1646-9.
6-demethoxynobiletin, a nobiletin-analog citrus flavonoid, enhances extracellular signal-regulated kinase phosphorylation in PC12D cells.
We previously demonstrated that nobiletin, a polymethoxylated flavone isolated from citrus peels, has the potential to improve cognitive dysfunction in patients with Alzheimer's disease (AD). Recent studies suggest that the generation of intraneuronal amyloid-beta (Aβ) oligomers is an early event in the pathogenesis of AD. Aβ oligomers cause deficits in the regulation of the extracellular signal-regulated kinase (ERK) signaling which is critical for consolidation of the memory. Our previous studies revealed that nobiletin activated ERK signaling and subsequent cyclic AMP response element-dependent transcription.
METHODS AND RESULTS:
In this study, the effects of five nobiletin analogs, 6-demethoxynobiletin, tangeretin, 5-demethylnobiletin, sinensetin, and 6-Demethoxytangeretin, isolated from citrus peels were assessed on ERK phosphorylation in PC12D cells, and the structure-activity relationships were examined. PC12D cells were treated with nobiletin or its analogs, and the cell extracts were analyzed by Western blotting using an antibody specific to phosphorylated ERK. 6-Demethoxynobiletin markedly enhanced ERK phosphorylation in a concentration-dependent manner.
CONCLUSIONS:
These results may be useful in developing drugs and functional foods using citrus peels for the treatment of dementia including AD.
J Rheumatol. 2000 Jan;27(1):20-5.
A citrus flavonoid, nobiletin, suppresses production and gene expression of matrix metalloproteinase 9/gelatinase B in rabbit synovial fibroblasts.
Flavonoids including nobiletin are known to exert many biological actions in vitro. We investigated the chondroprotective effect of citrus flavonoids, especially nobiletin, using cultured rabbit synovial fibroblasts and articular chondrocytes.
METHODS AND RESULTS:
We examined the effects of citrus flavonoids on the production and gene expression of matrix metalloproteinases (MMP) and prostaglandin E2 (PGE2)production in rabbit synovial fibroblasts. Six flavonoids isolated from Citrus depressa Rutaceae including tangeretin, 6-Demethoxytangeretin, nobiletin, 5-demethylnobiletin, 6-demethoxynobiletin, and sinensetin suppressed the interleukin 1 (IL-1) induced production of proMMP-9/progelatinase B in rabbit synovial cells in a dose dependent manner (<64 microM); nobiletin most effectively suppressed proMMP-9 production along with the decrease in its mRNA. Nobiletin also reduced IL-1 induced production of PGE2 in the synovial cells, but did not modify the synthesis of total protein. These suppressive effects of nobiletin were also observed in rabbit articular chondrocytes. Nobiletin inhibited proliferation of rabbit synovial fibroblasts in the growth phase.
CONCLUSIONS:
These results suggest nobiletin is a novel antiinflammatory candidate that has the potential to inhibit PGE2 production, matrix degradation of the articular cartilage, and pannus formation in osteoarthritis and rheumatoid arthritis. CONCLUSION: These results suggest nobiletin is a novel antiinflammatory candidate that has the potential to inhibit PGE2 production, matrix degradation of the articular cartilage, and pannus formation in osteoarthritis and rheumatoid arthritis.
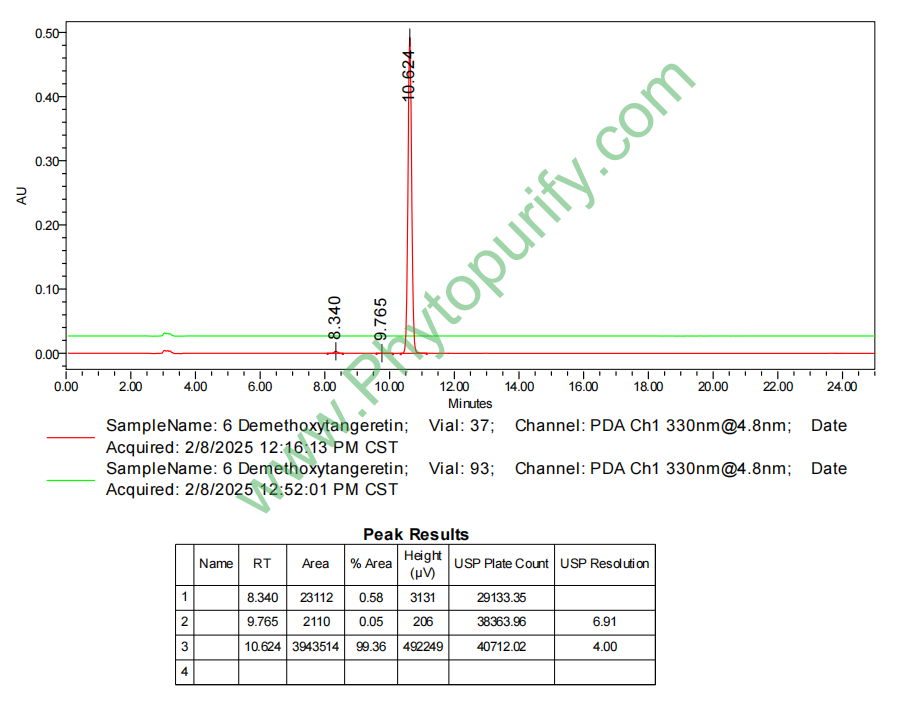
HPLC of 6-Demethoxytangeretin
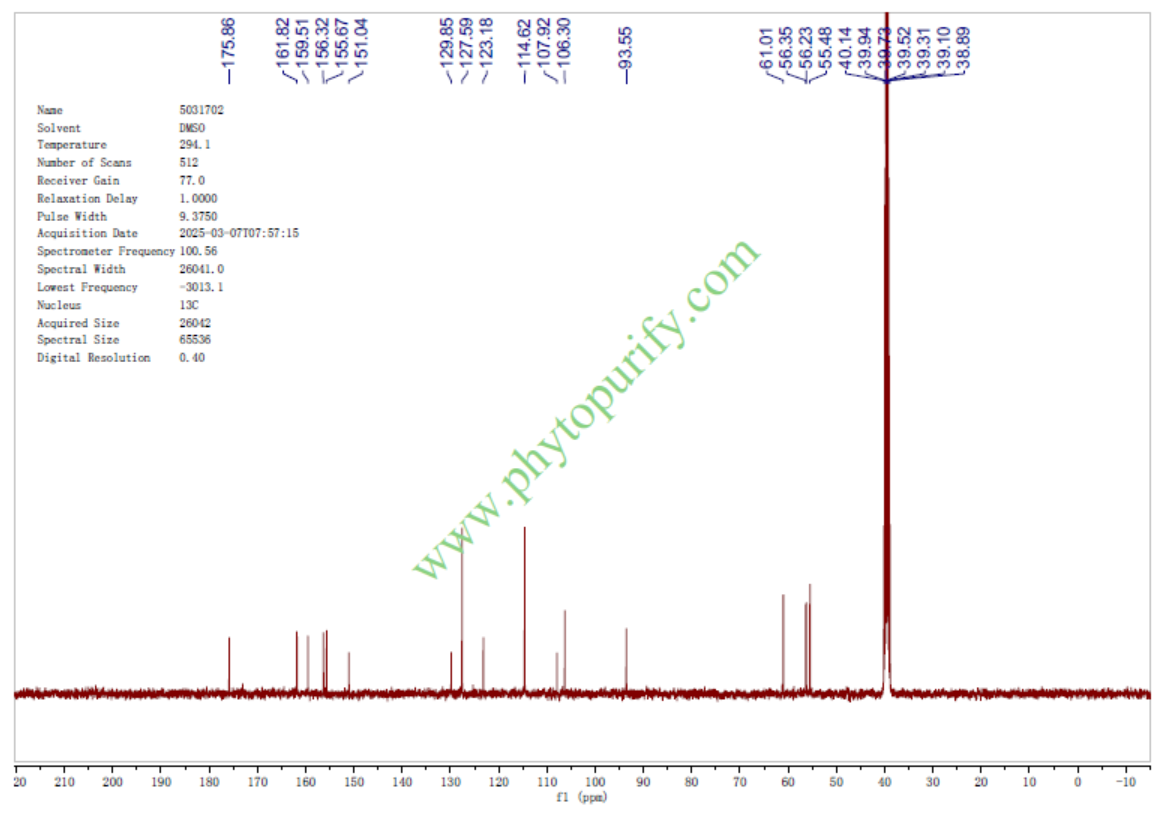
NMRof 6-Demethoxytangeretin