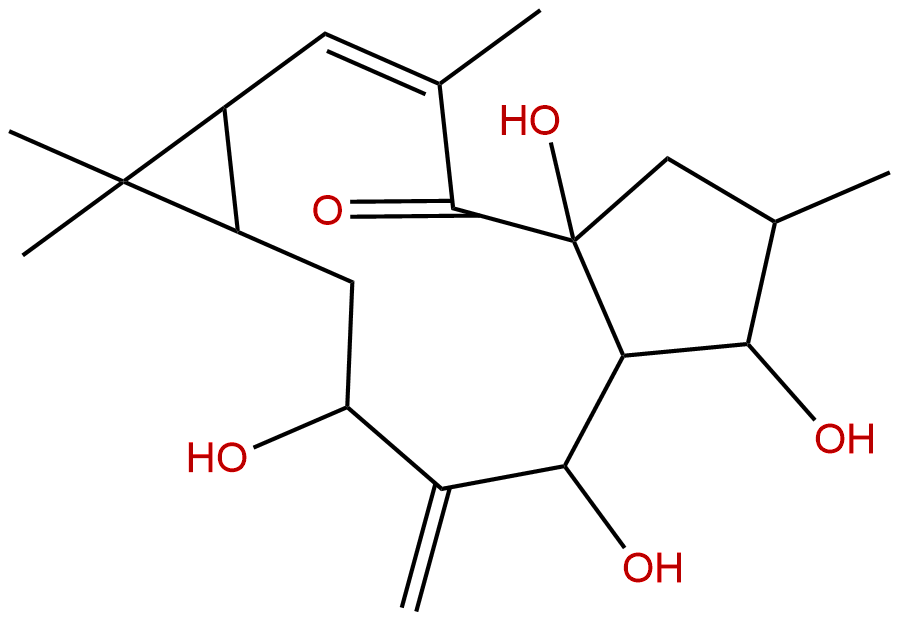
7-beta-HydroxylathyrolCAS No.:34208-98-5
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0097 |
| Formula: | C20H30O5 |
| Mol Weight: | 350.455 |
Synonym name: 7-Hydroxylathyrol
Catalogue No.: BP0097
Cas No.: 34208-98-5
Formula: C20H30O5
Mol Weight: 350.455
Botanical Source: Euphorbia lathyris
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Description:
Reference standards.
References:
Journal of Molecular Catalysis B: Enzymatic, 2017:S1381117717300279.
Regioselective hydroxylation of lathyrane diterpenoids biocatalyzed by microorganisms and its application in integrated synthesis.
METHODS AND RESULTS:
The regioselective oxidation of three lathyrane diterpenoids (lathyrol, 1; 7-beta-Hydroxylathyrol, 2; and Euphorbia factor L3, 3) catalyzed by the fungi Mortierella ramanniana CGMCC 3.03413, Mucor circinelloides CICC 40242, and the actinomycete Nocardia iowensis sp. nov. NRRL 5646 was investigated. Ten new metabolites (4–13) including two unprecedented cyclopropane-rearranged products (4 and 5) have been obtained. Metabolites (6, 10 and 11) were further converted chemically to eight acylated derivatives (6a, 10a–10f and 11a).
CONCLUSIONS:
The structures of all compounds were elucidated on the basis of extensive NMR and MS data analyses. All the metabolites and enzyme-chemical products were evaluated for their cytotoxicities against three human cancer cell lines as well as their multidrug resistance (MDR) reversing effects in adriamycin (ADM)-resistant human MCF-7 breast cancer cells (MCF-7/ADM).
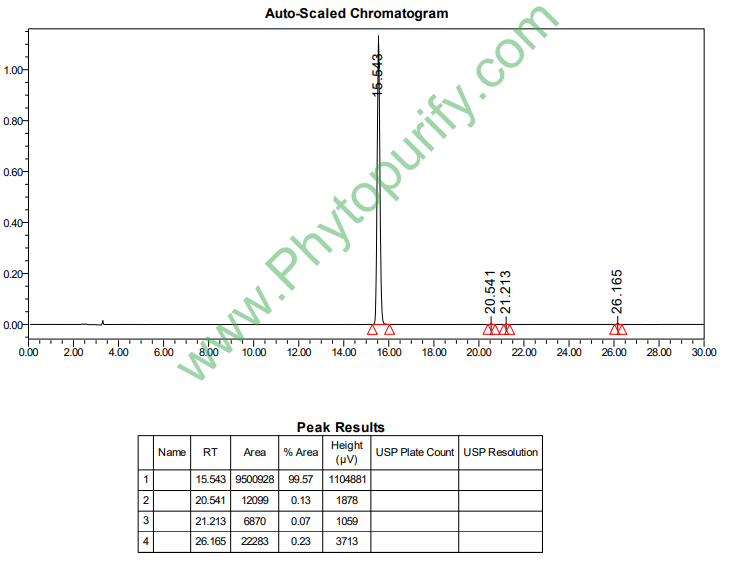
HPLC of 7-beta-Hydroxylathyrol