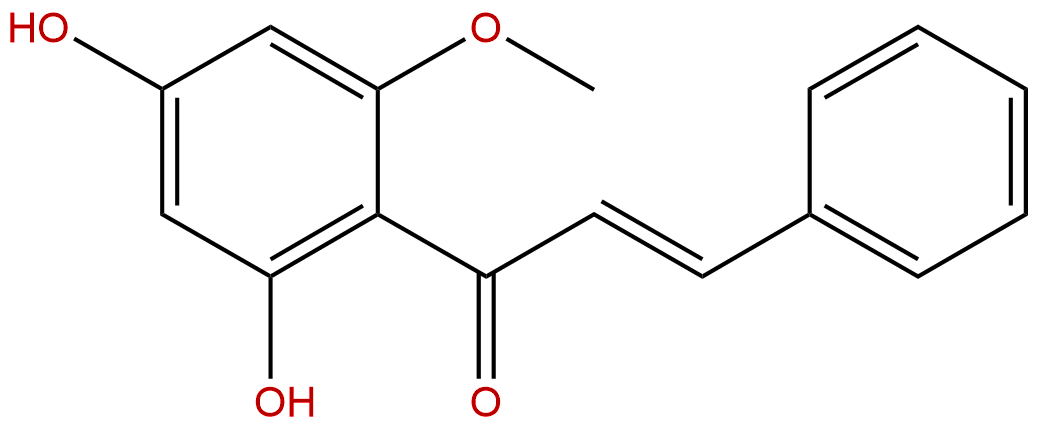
CardamoninCAS No.:19309-14-9
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0315 |
| Formula: | C16H14O4 |
| Mol Weight: | 270.284 |
Product name: Cardamonin
Synonym name: 2′,4′-Dihydroxy-6′-methoxychalcone; Alpinetin chalcone
Catalogue No.: BP0315
Cas No.: 19309-14-9
Formula: C16H14O4
Mol Weight: 270.284
Botanical Source: Alpinia katsumadai Hayata
Physical Description:
Type of Compound: Chalcones
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Cardamonin is a naturally occurring chalcone with strong anti-inflammatory , anticancer, anti-melanogenesis, and vascular activity. It is a novel TRPA1 antagonist with IC50 of 454 nM and also a NF-kB inhibitor. It ameliorates insulin resistance induced by high insulin and high glucose through the mTOR and signal pathway. It also may be a potential whitening agent for use in cosmetics and in the medical treatment of hyperpigmentation disorders.
References:
Biomol Ther (Seoul). 2015 Mar;23(2):141-8.
Cardamonin Suppresses TGF-β1-Induced Epithelial Mesenchymal Transition via Restoring Protein Phosphatase 2A Expression.
Epithelial mesenchymal transition (EMT) is the first step in metastasis and implicated in the phenotype of cancer stem cells. Therefore, understanding and controlling EMT, are essential to the prevention and cure of metastasis.
METHODS AND RESULTS:
In the present study, we examined, by Western blot, reverse transcription polymerase chain reaction (RT-PCR), and confocal microscopy, the effects of Cardamonin (CDN) on transforming growth factor-β1 (TGF-β1)-induced EMT of A549 lung adenocarcinoma cell lines. TGF-β1 induced expression of N-cadherin and decreased expression of E-cadherin. CDN suppressed N-cadherin expression and restored E-cadherin expression. Further, TGF-β1 induced migration and invasion of A549 cancer cells, which was suppressed by CDN. TGF-β1 induced c-Jun N-terminal kinase (JNK) activation during EMT, but CDN blocked it. Protein serine/threonine phosphatase 2A (PP2A) expression in A549 cancer cells was reduced by TGF-β1 but CDN restored it.
METHODS AND RESULTS:
The overall data suggested that CDN suppresses TGF-β1-induced EMT via PP2A restoration, making it a potential new drug candidate that controls metastasis.
Mol Immunol. 2007 Feb;44(5):673-9.
Cardamonin inhibits COX and iNOS expression via inhibition of p65NF-kappaB nuclear translocation and Ikappa-B phosphorylation in RAW 264.7 macrophage cells.
Cardamonin, a chalcone isolated from the fruits of a local plant Alpinia rafflesiana, has Cardamonin, a chalcone isolated from the fruits of a local plant Alpinia rafflesiana, has demonstrated anti-inflammatory activity in cellular models of inflammation.
METHODS AND RESULTS:
In this report, we evaluated the ability of Cardamonin to suppress both NO and PGE2 synthesis, iNOS and COX-2 expression and enzymatic activity, and key molecules in the NF-kappaB pathway in order to determine its molecular target. Cardamonin suppressed the production of NO and PGE2 in interferon-gamma (IFN-gamma)- and lipopolysaccharide (LPS)-induced RAW 264.7 cells. This inhibition was demonstrated to be caused by a dose-dependent down-regulation of both inducible enzymes, iNOS and COX-2, without direct effect upon iNOS or COX-2 enzyme activity. Subsequently we determined that the inhibition of inducible enzyme expression was due to a dose-dependent inhibition of phosphorylation and degradation of I-kappaBalpha, which resulted in a reduction of p65NF-kappaB nuclear translocation.
CONCLUSIONS:
We conclude that Cardamonin is a potential anti-inflammatory drug lead that targets the NF-kappaB pathway.
J Cardiovasc Pharmacol. 2001 May;37(5):596-606.
Vasorelaxant effects of cardamonin and alpinetin from Alpinia henryi K. Schum.
The vascular effects of Cardamonin and alpinetin from Alpinia henryi K. Schum. were examined in the rat isolated mesenteric arteries.
METHODS AND RESULTS:
1H and 13C nuclear magnetic resonance spectra showed that Cardamonin is present in trans-form, and single-crystal radiographic structure revealed that alpinetin is present in S configuration. Both Cardamonin and alpinetin produced a rightward shift in the concentration-response curve for phenylephrine in a noncompetitive manner, and they induced relaxation of phenylephrine-preconstricted arteries with respective mean inhibitory concentrations (IC50) of 9.3+/-0.6 microM and 27.5+/-2.8 microM. Both compounds also relaxed arteries preconstricted by endothelin I or U46619. Their relaxant effects were decreased in endothelium-removed rings. Pretreatment with N(G)-nitro-L-arginine methyl ester or methylene blue inhibited relaxation induced by both agents, and pretreatment with L-arginine reversed the effect of N(G)-nitro-L-arginine methyl ester on Cardamonin-induced endothelium-dependent relaxation. The relaxant effects of Cardamonin and alpinetin were unaffected by indomethacin (3 microM). Cardamonin and alpinetin inhibited 60 mM K+-induced contraction with respective IC50 of 11.5+/-0.3 microM and 37.9+/-3.6 microM. In addition, both agents inhibited the transient contraction induced by 3 microM phenylephrine or by 10 mM caffeine in Ca2+-free Krebs solution. Finally, these two agents also concentration dependently relax the arteries preconstricted by 1 microM phorbol 12,13-diacetate in Ca2+-free Krebs solution.
CONCLUSIONS:
These results indicate that purified Cardamonin and alpinetin from A. henryi K. Schum. relaxed rat mesenteric arteries through multiple mechanisms. They induced both endothelium-dependent and -independent relaxation; the former is likely mediated by nitric oxide whereas the latter is probably mediated through nonselective inhibition of Ca2+ influx and intracellular Ca2+ release and inhibition of the protein kinase C-dependent contractile mechanism.
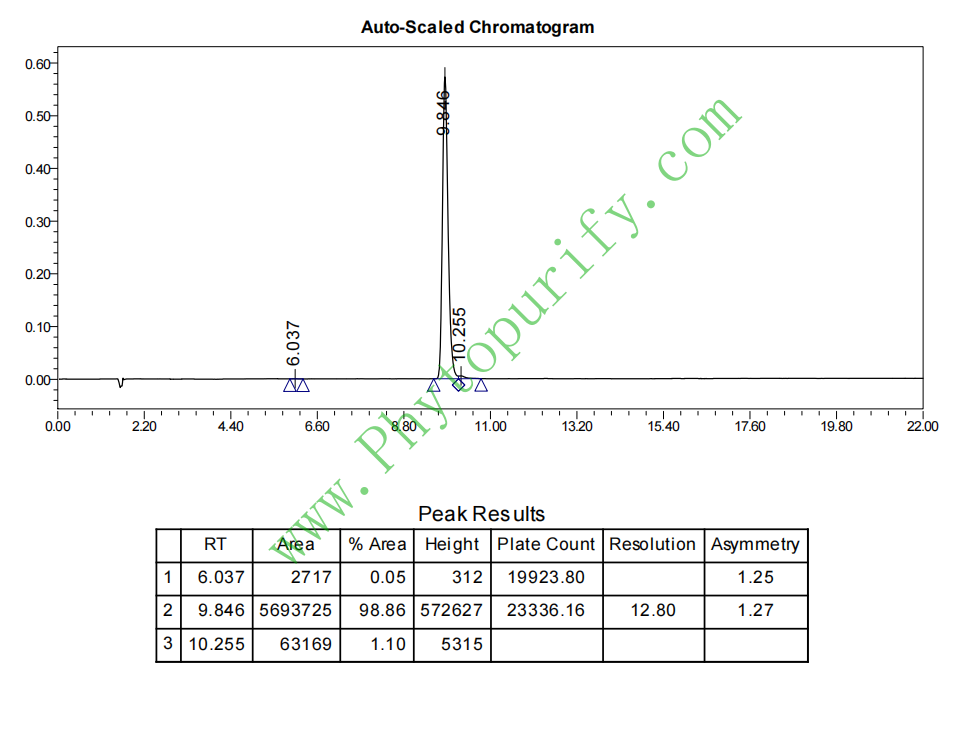
HPLC of Cardamonin