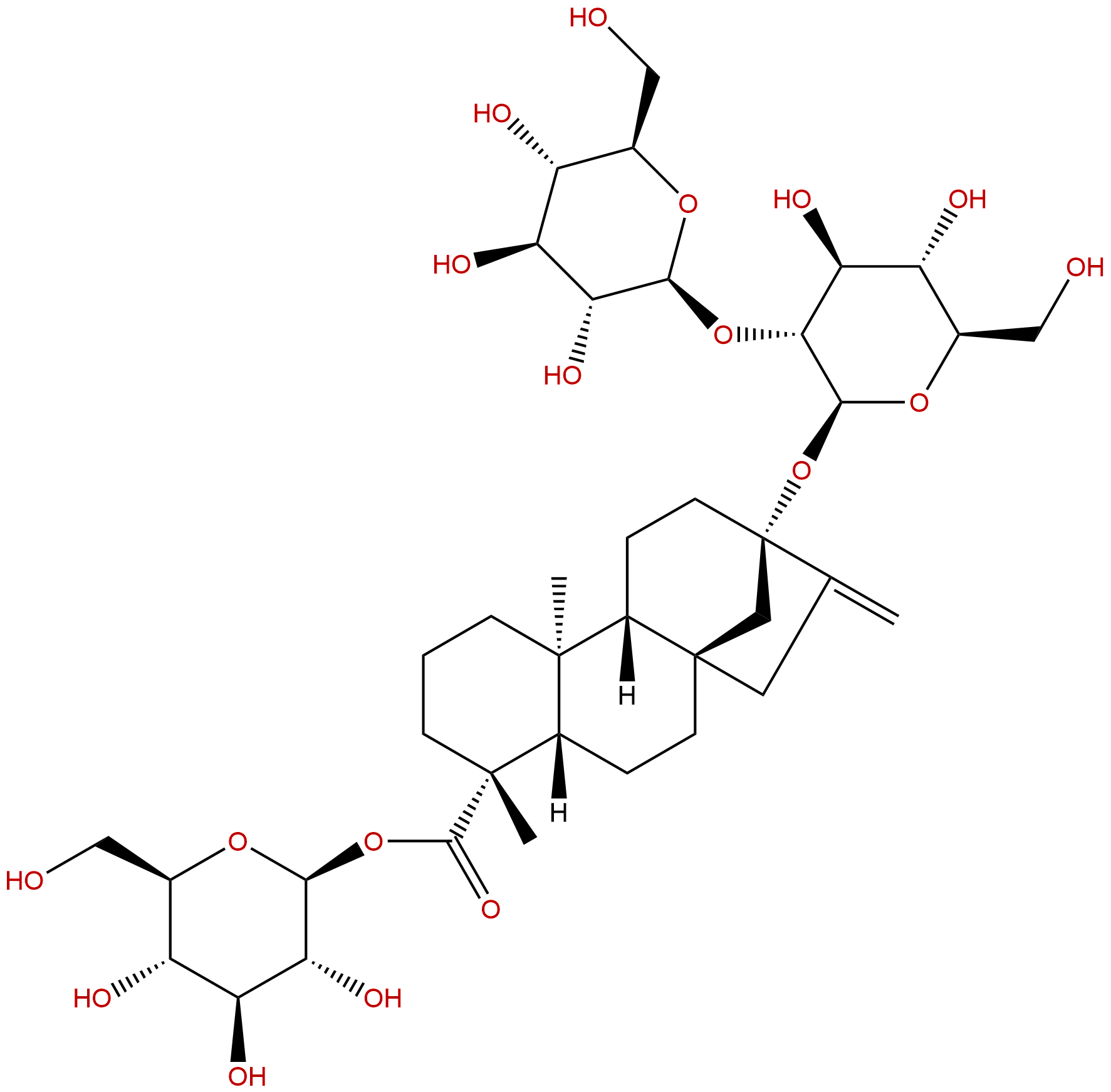
SteviosideCAS No.:57817-89-7
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1342 |
| Formula: | C38H60O18 |
| Mol Weight: | 804.88 |
Product name: Stevioside
Synonym name: Eupatorin; Rebaudin; Stevin; Steviosin
Catalogue No.: BP1342
Cas No.: 57817-89-7
Formula: C38H60O18
Mol Weight: 804.88
Botanical Source: Stevia rebaudiana (Bertoni) Hemsl.
Physical Description:
Type of Compound: Diterpenoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Stevioside is a safe natural sweetener, has no allergic reactions, suited for both diabetics, and PKU patients, as well as for obese persons intending to lose weight by avoiding sugar supplements in the diet. Stevioside enjoys a dual positive effect by acting as an antihyperglycemic and a blood pressure-lowering substance, it may have therapeutic potential in the treatment of type 2 diabetes and the metabolic syndrome.Stevioside exerts anti-inflammatory and anti-apoptotic properties by inhibiting the release of cytokines and the activation of TLR2 and proteins of the NF-κB and MAPK signaling pathways, as well as caspase-3 and Bax.
References:
Int Immunopharmacol. 2014 Sep;22(1):192-9.
Stevioside inhibits inflammation and apoptosis by regulating TLR2 and TLR2-related proteins in S. aureus-infected mouse mammary epithelial cells.
METHODS AND RESULTS:
The object of this study was to investigate the anti-inflammatory and anti-apoptosis function of Stevioside and the possible molecular mechanisms for such activity in Staphylococcus aureus (S. aureus)-infected mouse mammary epithelial cells (MMECs). The cells were treated with varying doses of Stevioside before infection with S. aureus. The live/dead cells were detected by immunofluorescence microscopy. The pro-inflammatory cytokines were determined by ELISA. The mRNA of TLR2 and proteins related to NF-κB, MAPK and apoptosis were analyzed by q-PCR. The relative protein expression levels were determined by Western blot. The results indicated that Stevioside inhibited the mRNA and protein expression of TNF-α, IL-6 and IL-1β dose-dependently in S. aureus-stimulated MMECs. Stevioside suppressed the S. aureus-induced expression of TLR2 and proteins of the NF-κB and MAPK pathways as well as apoptosis. The mRNA levels of IκBα, p38, ERK, JNK, p65, caspase-3 and Bax were not influenced by the Stevioside treatment.
CONCLUSIONS:
Stevioside exerts anti-inflammatory and anti-apoptotic properties by inhibiting the release of cytokines and the activation of TLR2 and proteins of the NF-κB and MAPK signaling pathways, as well as caspase-3 and Bax.
Braz Oral Res. 2014 Jan-Feb;28(1).
Effects of lactose-containing stevioside sweeteners on dental biofilm acidogenicity.
The aim of this study was to evaluate the effect of a commercial lactose-containing Stevioside sweetener on biofilm acidogenicity in vivo.
METHODS AND RESULTS:
Nine volunteers refrained from brushing their teeth for 3 days in five phases. On the 4th day of each phase, the pH of the biofilm was measured by the "Strip method". Interproximal plaque pH was measured before and up to 60 minutes after a 10 mL mouthrinse for 1 minute with the test solutions: I - sweetener with 93% lactose and 7% Stevioside; II - sweetener with 6.8% saccharin, 13.6% cyclamate, and 0.82% Stevioside; III - 18% sucrose solution (positive control); IV - mineral water (negative control); and V- 93% lactose solution. The results revealed that the most pronounced pH fall was found with sucrose (positive control), followed by the 93% lactose solution, the sweetener with lactose + Stevioside, the sweetener with saccharin + cyclamate + Stevioside, and finally water (negative control). According to the area under the curve, the two sweeteners containing Stevioside were significantly different, and the sweetener with lactose + Stevioside was significantly different from water but not from sucrose. The critical pH for dentin demineralization (pH ≤ 6.5) was reached by all volunteers after rinsing with sucrose solution, lactose solution, and the Stevioside+ lactose sweetener.
CONCLUSIONS:
Analysis of the data suggests that lactose-containing Stevioside sweeteners may be cariogenic, especially to dentin.
Metabolism. 2003 Mar;52(3):372-8.
Antihyperglycemic and blood pressure-reducing effects of stevioside in the diabetic Goto-Kakizaki rat.
Stevioside, a glycoside present in the leaves of the plant, Stevia rebaudiana Bertoni (SrB), has acute insulinotropic effects in vitro. Its potential antihyperglycemic and blood pressure-lowering effects were examined in a long-term study in the type 2 diabetic Goto-Kakizaki (GK) rat.
METHODS AND RESULTS:
Rats were fed 0.025 g x kg(-1) x d(-1) of Stevioside (purity > 99.6%) for 6 weeks. An intra-arterial catheter was inserted into the rats after 5 weeks, and conscious rats were subjected to arterial glucose tolerance test (2.0 g x kg(-1)) during week 6. Stevioside had an antihyperglycemic effect (incremental area under the glucose response curve [IAUC]): 985 +/- 20 (Stevioside) versus 1,575 +/- 21 (control) mmol/L x 180 minutes, (P <.05), it enhanced the first-phase insulin response (IAUC: 343 +/- 33 [Stevioside] v 136 +/- 24 [control] microU/mL insulin x 30 minutes, P <.05) and concomitantly suppressed the glucagon levels (total AUC: 2,026 +/- 234 [Stevioside] v 3,535 +/- 282 [control] pg/mL x 180 minutes, P <.05). In addition, Stevioside caused a pronounced suppression of both the systolic (135 +/- 2 v 153 +/- 5 mm Hg; P <.001) and the diastolic blood pressure (74 +/- 1 v 83 +/- 1 mm Hg; P <.001). Bolus injections of Stevioside (0.025 g x kg(-1)) did not induce hypoglycemia. Stevioside augmented the insulin content in the beta-cell line, INS-1. Stevioside may increase the insulin secretion, in part, by induction of genes involved in glycolysis. It may also improve the nutrient-sensing mechanisms, increase cytosolic long-chain fatty acyl-coenzyme A (CoA), and downregulate phosphodiesterase 1 (PDE1) estimated by the microarray gene chip technology.
CONCLUSIONS:
In conclusion, Stevioside enjoys a dual positive effect by acting as an antihyperglycemic and a blood pressure-lowering substance; effects that may have therapeutic potential in the treatment of type 2 diabetes and the metabolic syndrome.
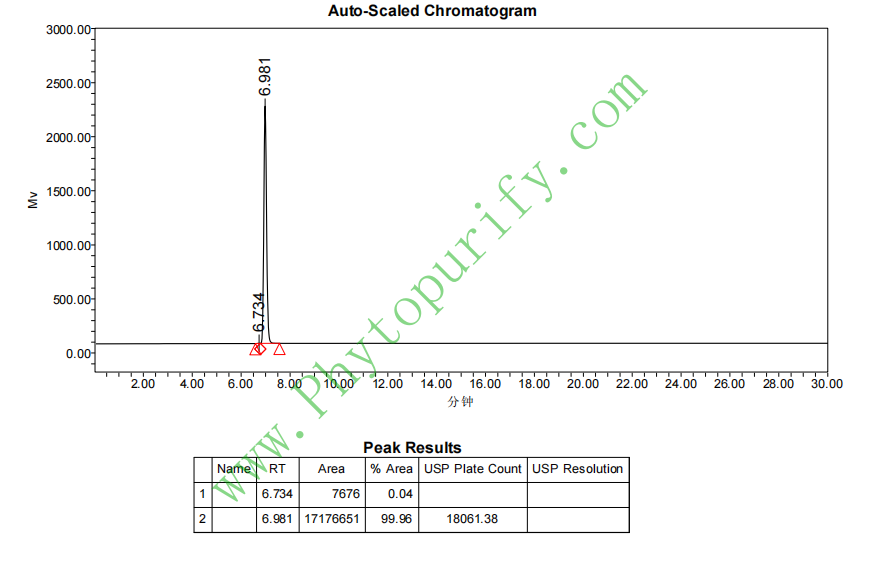
HPLC of Stevioside