
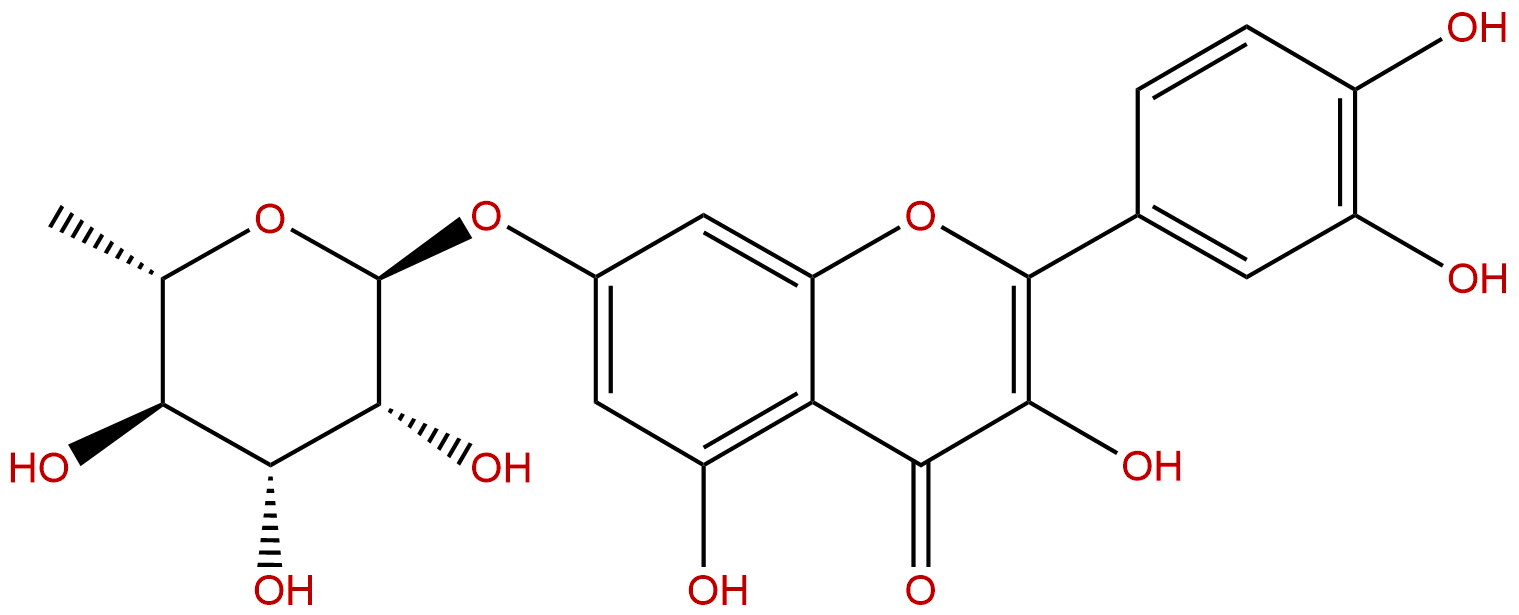
Quercetin 7-rhamnosideCAS No.:22007-72-3
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1189 |
| Formula: | C21H20O11 |
| Mol Weight: | 448.38 |
Synonym name: Vincetoxicoside B; -Rhamnosylquercetin
Catalogue No.: BP1189
Cas No.: 22007-72-3
Formula: C21H20O11
Mol Weight: 448.38
Botanical Source: Vincetoxicum officinale, Sedum caucasicum and other plant spp.
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Description:
Vincetoxicoside B , quercetin, kaempferol , and (-)-epicatechin show synergistic antifungal activities with the FICI values <0.5.
References:
J Asian Nat Prod Res. 2017 Jan;19(1):47-52.
Chemical constituents from the rhizome of Polygonum paleaceum and their antifungal activity.
METHODS AND RESULTS:
A new compounds neopaleaceolactoside (1), along with nine known compounds phyllocoumarin (2), quercetin (3), quercitrin (4), quercetin-3-methyl ether (5), Vincetoxicoside B (6), isoquercitrin (7), kaempferol (8), (-)-epicatechin (9), and chlorogenic acid (10), was isolated from Polygonum paleaceum Wall. Their chemical structures were established based on one-dimensional and two-dimensional nuclear magnetic resonance techniques, mass spectrometry and by comparison with spectroscopic data reported. Some selected compounds were screened for their antifungal activity. Quercetin (3), Vincetoxicoside B (6), kaempferol (8), and (-)-epicatechin (9) showed synergistic antifungal activities with the FICI values <0.5.
CONCLUSIONS:
A preliminary structure-activity relationship could be observed that free 3-OH in the structure of flavonoids was important for synergistic antifungal activity.
HPLC of Quercetin 7-rhamnoside
